Abstract
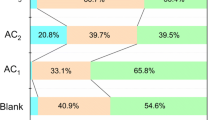
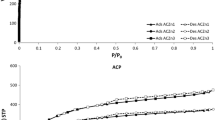
Flue gas pollution is a serious environmental problem that needs to be solved for the sustainable development of China. The surface chemical properties of carbon have great influence on its desulphurization performance. A series of activated carbons (ACs) were prepared using HNO3, H2O2, NH3·H2O and steam as activation agents with the aim to introduce functional groups to carbon surface in the ACs preparation process. The ACs were physically and chemically characterized by iodine and SO2 adsorption, ultimate analysis, Boehm titration, and temperature-programmed reduction (TPR). Results showed that the iodine number and desulphurization capacity of NH3·H2O activated carbon (AC-NH3) increase with both activation time, and its desulphurization capacity also increases with the concentration of activation agent. However, HNO3 activated carbon (AC-HNO3) and H2O2 activated carbon (AC-H2O2) exhibit more complex behavior. Only their iodine numbers increase monotonously with activation time. Compared with steam activated AC (AC-H2O), the nitrogen content increases 0.232% in AC-NH3 and 0.077% in AC-HNO3. The amount of total basic site on AC-HNO3 is 0.19 mmol·g−1 higher than that on AC-H2O. H2O2 activation introduces an additional 0.08 mmol·g−1 carboxyl groups to AC surface than that introduced by steam activation. The desulphurization capacity of ACs in simulate flue gas desulphurization decreases as follows: AC-NH3 > AC-HNO3 > AC-H2O2 > AC-H2O. This sequence is in accord with the SO2 catalytic oxidation/oxidation ratio in the absence of oxygen and the oxidation property reflected by TPR. In the presence of oxygen, all adsorbed SO2 on ACs can be oxidized into SO3. The desulphurization capacity increases differently according to the activation agents; the desulphurization capacity of AC-NH3 and AC-HNO3 improves by 4.8 times, yet AC-H2O increases only by 2.62 as compared with the desulphurization of corresponding ACs in absence of oxygen.
Similar content being viewed by others
References
Sumathi S, Bhatia S, Lee K T, et al. Performance of an activated carbon made from waste palm shell in simultaneous adsorption of SOx and NOx of flue gas at low temperature. Sci China Ser E-Tech Sci, 2009, 52(1): 198–203
Zhao Y, Sun X J, Xu P Y, et al. Mechanism of flue gas simultaneous desulfurization and denitrification using the highly reactive absorbent. Sci China Ser E-Tech Sci, 2005, 48(6): 692–705
Zou C, Huang Z J, Xiong J, et al. A pilot scale study on co-capture of SO2 and NOx in O2/CO2 recycled coal combustion and techno-economic evaluation. Sci China Tech Sci, 2010, 53: 155–159
Zhang Y Q, Fang Y T, Huang J J, et al. Effects of micropore structure and surface properties on the SO2 removal in flue gas by active coke (in Chinese). J Combust Sci Technol, 2004, 10(2): 160–164
Zhang X L, Zhang Y, Ding F S, et al. Effect of the surface properties of an activated coke on its desulphurization performance. Min Sci Technol, 2009, 19(6): 769–774
Davini P. Adsorption and desorption of SO2 on active carbon: The effect of surface basic groups. Carbon, 1990, 28(4): 565–571
Davini P. Influence of surface properties and iron addition on the SO2 adsorption capacity of activated carbons. Carbon, 2002, 40(5): 729–734
Xu L S, Guo J, Jin F, et al. Removal of SO2 from O2-containing flue gas by activated carbon fiber (ACF) impregnated with NH3. Chemosphere, 2006, 62(5): 823–826
Wang Q, Xiao Y L, Qiao W M, et al. Modification of polystyrene-based activeated carbon spheres to improve adsorption of dibenzothiophene. Appl Surf Sci, 2009, 255: 3499–3506
Christian L, Mangun K, Benak R, et al. Surface chemistry pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon, 2001, 39: 1809–1820
Shim J W, Park S J, Ryu S K. Effect of modification with HNO3 and NaOH on metal adsorption by pitch-based activated carbon fibers. Carbon, 2001, 39(11): 1635–1642
Abdel-Nasser A, Hendawy E l. Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon, 2003, 41(4): 713–722
Boehm H P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon, 1994, 32(5): 759–769
Zhang X L, Liu Y, Li K, et al. Effect of activated char preparation conditions on its desulphurization property (in Chinese). Coal Convers, 2007, 30(4): 51–54
Luiz C A, Oliveira C N S, Maria I Y, et al. The effect of H2 treatment on the activity of activated carbon for the oxidation of organic contaminants in water and the H2O2 decomposition. Carbon, 2004, 42: 2279–2284
Calo J M, Cazorla-Amoros D, Linares-Solano A, et al. The effect of hydrogen on the thermal desorption of oxygen surface complexes. Carbon, 1997, 35(4): 543–544
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Zhang, Y., Wang, S. et al. Effect of activation agents on the surface chemical properties and desulphurization performance of activated carbon. Sci. China Technol. Sci. 53, 2515–2520 (2010). https://doi.org/10.1007/s11431-010-4058-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-010-4058-5