Abstract
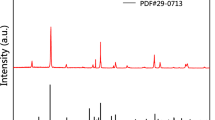
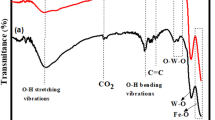
As other natural iron-bearing minerals, schorl could be taken as an effective iron source for degradation of organic pollutants by mineral-catalyzed Fenton-like system. In our present study, the schorl-catalyzed Fenton-like system has been successfully developed for discoloration of an active commercial dye, Rhodamine B (RhB), in an aqueous solution. Through a number of batch discoloration experiments under various conditions, it was found that the reactivity of the system increased by, respectively, increasing schorl dosage, temperature, hydrogen peroxide starting concentration and by decreasing the pH. Over 90% of discoloration ratio could be gained in less than 30 min, and nearly 70% of total organic carbon (TOC) could be removed in less than 200 min. And, the schorl catalyst could be repeatedly used at least ten times, still with high catalytic activity. Comparative studies indicated that the RhB discoloration ratios were much higher in presence of schorl and H2O2 than those in presence of schorl or H2O2 only, which suggested that the schorl-catalyzed Fenton-like reaction governed the RhB discoloration process. The content of Fe ion leaching in the solution was also measured using inductively coupling plasma-atomic emission spectra (ICP-AES). A mechanism proposed herein suggested that adsorption and Fenton-like reaction (heterogeneous and homogeneous) were responsible for the discoloration of RhB.
Similar content being viewed by others
References
Malik P K. Oxidation of Safranine T in Aqueous Solution Using Fenton’s Reagent: Involvement of an Fe (III) Chelate in the Catalytic Hydrogen Peroxide Oxidation of Safranine T. J Phys Chem A, 2004, 108: 2675–2681
Duesterberg C K, Waite T D. Process Optimization of Fenton Oxidation Using Kinetic Modeling. Environ Sci Technol, 2006, 40: 4189–4195
Leung Y F. Development of MCM-41 based catalysts for the photo-Fenton’s degradation of dye pollutants. Doctoral Dissertation. Hong Kong: The Hong Kong University of Science and Technology, 2005. 8–10
Schoonen M A A, Xu Y, Strongin D R. An introduction to geocatalysis. J Geochem Explor, 1998, 62: 201–215
Kwan W, Voelker B. Rates of hydroxyl radical generation and organic compound oxidation in mineral-catalyzed Fenton-like systems. Environ Sci Technol, 2003, 37: 1150–1158
Matta R, Hanna K, Chiron S. Fenton-like oxidation of 2,4,6-trinitrotoluene using different iron minerals. Sci Total Environ, 2007, 385: 242–251
Yeh C K-J, Hsu C-Y, Chiu C-H, et al. Reaction efficiencies and rate constants for the goethite-catalyzed Fenton-like reaction of NAPL-form aromatic hydrocarbons and chloroethylenes. J Hazard Mater, 2008, 151: 562–569
Andreozzi R, Canterino M, Caprio V, et al. Use of an amorphous iron oxide hydrated as catalyst for hydrogen peroxide oxidation of ferulic acid in water. J Hazard Mater, 2008, 152: 870–875
Zhao Y P, Hu J Y. Photo-Fenton degradation of 17β-eatradiol in presence of α-FeOOHR and H2O2. Appl Catal, B: Environ, 2008, 78: 250–258
Liou M-J, Lu M-C. Catalytic degradation of explosives with goethite and hydrogen peroxide. J Hazard Mater, 2008, 151: 540–546
Barreiro J C, Capelato M D, Martin-Neto L, et al. Oxidative decomposition of atrazine by a Fenton-like reaction in a H2O2/ferrihydrite system. Water Res, 2007, 41: 55–62
Barton R J. Refinement of the crystal structure of buergerite and the absolute orientation of schorl. Acta Crystallogr, 1969, B25: 1524–1533
Yavuza F, Gültekin A H, Karakaya M C. CLASTOUR: a computer program for classification of the minerals of the tourmaline group. Comput Geosci, 2002, 28: 1017–1036
Prasad P S R, Srinivasa D S. Study of structural disorder in natural tourmalines by infrared spectroscopy. Gondwana Res, 2005, 8: 265–270
Jin Z Z, Ji Z J, Liang J S, et al. Observation of spontaneous polarization of tourmaline. Chin Phys, 2003, 12: 222–225
Nakamura T, Fujishiro K, Kubo T, et al. Tourmaline and lithium niobate reaction with water. Ferroelectrics, 1994, 155: 207–212
Xia M S, Hu C H, Zhang H M. Effects of tourmaline addition on the dehydrogenase activity of Rhodopseudomonas palustris. Process Biochem, 2006, 41: 221–225
Jiang K, Sun T H, Sun L N, et al. Adsorption characteristics of copper, lead, zinc and cadmium ions by tourmaline. J Environ Sci, 2006, 18: 1221–1225
Meng J P, Liang J S, Ou X Q, et al. Effects of mineral tourmaline particles on the photocatalytic activity of TiO2 thin films. J Nanosci Nanotechnol, 2008, 8: 1279–1283
Liang J S, Zhu D B, Meng J P, et al. Performance and application of far infrared rays emitted from rare earth mineral composite materials. J Nanosci Nanotechnol, 2008, 8: 1203–1210
Oishi T. Water drinker container for dogs and cats has water activation material of natural raw material placed on central recess at container bottom. Japanese Patent, JP2005110522-A, 2005-04-28
Yang X, Liu W. Environmental protection type negative ion coating material and fabricating method. Chinese Patent, CN1563228-A, 2005-01-12
Feng J Y, Hu X J, Yue P L. Novel bentonite clay-based Fe-nanocomposite as a heterogeneous catalyst for photo-Fenton discoloration and mineralization of Orange II. Environ Sci Technol, 2004, 38: 269–275
Lin S S, Gurol M D. Catalytic decomposition of hydrogen peroxide on iron oxide kinetics, mechanism and implications. Environ Sci Technol, 1998, 32: 1417–1423
Lu M C, Chen J N, Huang H H. Role of goethite dissolution in the oxidation of 2-chlorophenol with hydrogen peroxide. Chemosphere, 2002, 46: 131–136
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Heilongjiang Science Fundation for Young Scholars (Grant No. QC07C02) and Scientific Foundation of Heilongjiang Education Department, China (Grant No. 11531035)
Rights and permissions
About this article
Cite this article
Xu, H., Prasad, M., He, X. et al. Discoloration of Rhodamine B dyeing wastewater by schorl-catalyzed Fenton-like reaction. Sci. China Ser. E-Technol. Sci. 52, 3054–3060 (2009). https://doi.org/10.1007/s11431-009-0304-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-009-0304-0