Abstract
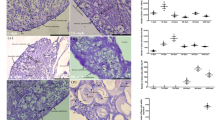
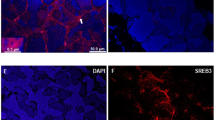
The role of glucocorticoids in oogenesis remains to be elucidated. cyp11c1 encodes the key enzyme involved in the synthesis of cortisol, the major glucocorticoid in teleosts. In our previous study, we mutated cyp11c1 in tilapia and analyzed its role in spermatogenesis. In this study, we analyzed its role in oogenesis. cyp11c1+/− XX tilapia showed normal ovarian morphology but poor egg quality, as indicated by the mortality of embryos before 3 d post fertilization, which could be partially rescued by the supplement of exogenous cortisol to the mother fish. Transcriptome analyses revealed reduced expression of maternal genes in the eggs of the cyp11c1+/− XX fish. The cyp11c1−/− females showed impaired vitellogenesis and arrested oogenesis due to significantly decreased serum cortisol. Further analyses revealed decreased serum E2 level and expression of amh, an important regulator of follicular cell development, and increased follicular cell apoptosis in the ovaries of cyp11c1−/− XX fish, which could be rescued by supplement of either exogenous cortisol or E2. Luciferase assays revealed a direct regulation of cortisol and E2 on amh transcription via GRs or ESRs. Taken together, our results demonstrate that cortisol safeguards oogenesis by promoting follicular cell survival probably via Amh signaling.
Similar content being viewed by others
References
Bala, R., Singh, V., Rajender, S., and Singh, K. (2021). Environment, lifestyle, and female infertility. Reprod Sci 28, 617–638.
Bayne, S., Li, H., Jones, M.E.E., Pinto, A.R., van Sinderen, M., Drummond, A., Simpson, E.R., and Liu, J.P. (2011). Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell 2, 333–346.
Berg, H., Modig, C., and Olsson, P.E. (2004). 17beta-estradiol induced vitellogenesis is inhibited by cortisol at the post-transcriptional level in Arctic char (Salvelinus alpinus). Reprod Biol Endocrinol 2, 62.
Bobe, J., and Labbé, C. (2010). Egg and sperm quality in fish. Gen Comp Endocrinol 165, 535–548.
Breen, K.M., and Mellon, P.L. (2014). Influence of stress-induced intermediates on gonadotropin gene expression in gonadotrope cells. Mol Cell Endocrinol 385, 71–77.
Callanan, M., Kudo, N., Gout, S., Brocard, M., Yoshida, M., Dimitrov, S., and Khochbin, S. (2000). Developmentally regulated activity of CRM1/XPO1 during early Xenopus embryogenesis. J Cell Sci 113, 451–459.
Chou, C.H., and Chen, M.J. (2018). The effect of steroid hormones on ovarian follicle development. In: Vitamins and Hormones. New York: Academic Press. 155–175.
Craig, S., and Ozlem, Y. (2018). Vitellogenesis and yolk proteins, fish. In: Encyclopedia of Reproduction (Second Edition). New York: Academic Press. 266–277.
Durlinger, A.L.L., Gruijters, M.J.G., Kramer, P., Karels, B., Ingraham, H. A., Nachtigal, M.W., Uilenbroek, J.T.J., Grootegoed, J.A., and Themmen, A.P.N. (2002a). Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 143, 1076–1084.
Durlinger, A.L.L., Visser, J.A., and Themmen, A.P.N. (2002b). Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction 124, 601–609.
Faught, E., and Vijayan, M.M. (2018). Maternal stress and fish reproduction: the role of cortisol revisited. Fish Fish 19, 1016–1030.
Faught, E., Santos, H.B., and Vijayan, M.M. (2020). Loss of the glucocorticoid receptor causes accelerated ovarian ageing in zebrafish. Proc R Soc B 287, 20202190.
Goikoetxea, A., Todd, E.V., and Gemmell, N.J. (2017). Stress and sex: does cortisol mediate sex change in fish? Reproduction 154, R149–R160.
Hu, H., Miao, Y.R., Jia, L.H., Yu, Q.Y., Zhang, Q., and Guo, A.Y. (2019). AnimalTFDB 3.0: a comprehensive resource for annotation and prediction of animal transcription factors. Nucleic Acids Res 47, D33–D38.
Huang, T.J., and Shirley Li, P. (2001). Dexamethasone inhibits luteinizing hormone-induced synthesis of steroidogenic acute regulatory protein in cultured rat preovulatory follicles. Biol Reprod 64, 163–170.
Jalabert, B. (2005). Particularities of reproduction and oogenesis in teleost fish compared to mammals. Reprod Nutr Dev 45, 261–279.
Kala, M., and Nivsarkar, M. (2016). Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen Comp Endocrinol 225, 117–124.
Kayo, D., Zempo, B., Tomihara, S., Oka, Y., and Kanda, S. (2019). Gene knockout analysis reveals essentiality of estrogen receptor β1 (Esr2a) for female reproduction in medaka. Sci Rep 9, 8868.
Kim, D., Langmead, B., and Salzberg, S.L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12, 357–360.
Lethimonier, C., Flouriot, G., Valotaire, Y., Kah, O., and Ducouret, B. (2000). Transcriptional interference between glucocorticoid receptor and estradiol receptor mediates the inhibitory effect of cortisol on fish vitellogenesis. Biol Reprod 62, 1763–1771.
Li, M., Wu, F., Gu, Y., Wang, T., Wang, H., Yang, S., Sun, Y., Zhou, L., Huang, X., Jiao, B., et al. (2012). Insulin-like growth factor 3 regulates expression of genes encoding steroidogenic enzymes and key transcription factors in the Nile tilapia gonad. Biol Reprod 86.
Li, M., Yang, H., Zhao, J., Fang, L., Shi, H., Li, M., Sun, Y., Zhang, X., Jiang, D., Zhou, L., et al. (2014). Efficient and heritable gene targeting in tilapia by CRISPR/Cas9. Genetics 197, 591–599.
Li, M., Sun, Y., Zhao, J., Shi, H., Zeng, S., Ye, K., Jiang, D., Zhou, L., Sun, L., Tao, W., et al. (2015). A tandem duplicate of anti-Müllerian hormone with a missense SNP on the Y Chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet 11, e1005678.
Li, M., Liu, X., Dai, S., Xiao, H., Qi, S., Li, Y., Zheng, Q., Jie, M., Cheng, C.H.K., and Wang, D. (2020). Regulation of spermatogenesis and reproductive capacity by Igf3 in tilapia. Cell Mol Life Sci 77, 4921–4938.
Liu, X., Xiao, H., Jie, M., Dai, S., Wu, X., Li, M., and Wang, D. (2020). Amh regulate female folliculogenesis and fertility in a dose-dependent manner through Amhr2 in Nile tilapia. Mol Cell Endocrinol 499, 110593.
Livak, K.J., and Schmittgen, T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408.
Lubzens, E., Young, G., Bobe, J., and Cerdà, J. (2010). Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165, 367–389.
Lubzens, E., Bobe, J., Young, G., and Sullivan, C.V. (2017). Maternal investment in fish oocytes and eggs: the molecular cargo and its contributions to fertility and early development. Aquaculture 472, 107–143.
Maradonna, F., Gioacchini, G., Notarstefano, V., Fontana, C.M., Citton, F., Dalla Valle, L., Giorgini, E., and Carnevali, O. (2020). Knockout of the glucocorticoid receptor impairs reproduction in female zebrafish. Int J Mol Sci 21, 9073.
Miller, K.A., Kenter, L.W., Breton, T.S., and Berlinsky, D.L. (2019). The effects of stress, cortisol administration and cortisol inhibition on black sea bass (Centropristis striata) sex differentiation. Comp Biochem Physiol Part A Mol Integr Physiol 227, 154–160.
Mommsen, T.P., Vijayan, M.M., and Moon, T.W. (1999). Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9, 211–268.
Morinaga, C., Saito, D., Nakamura, S., Sasaki, T., Asakawa, S., Shimizu, N., Mitani, H., Furutani-Seiki, M., Tanaka, M., and Kondoh, H. (2007). The hotei mutation of medaka in the anti-Müllerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc Natl Acad Sci USA 104, 9691–9696.
Nesan, D., Kamkar, M., Burrows, J., Scott, I.C., Marsden, M., and Vijayan, M.M. (2012). Glucocorticoid receptor signaling is essential for mesoderm formation and muscle development in zebrafish. Endocrinology 153, 1288–1300.
Nesan, D., and Vijayan, M.M. (2013). Role of glucocorticoid in developmental programming: evidence from zebrafish. Gen Comp Endocrinol 181, 35–44.
Nesan, D., and Vijayan, M.M. (2016). Maternal cortisol mediates hypothalamus-pituitary-interrenal axis development in zebrafish. Sci Rep 6, 22582.
Neves, P.R., Natali, M.R.M., Ribeiro, R.P., Vargas, L., Maehana, K.R., and Marengoni, N.G. (2009). Morphological characteristics of ovarian development of two Nile tilapia (Oreochromis niloticus) strains in mixed-culture systems. Arq Bras Med Vet Zootec 61, 1173–1182.
Onitake, K. (1972). Morphological studies of normal sex-differentiation and induced sex-reversal process of gonads in the medaka, Oryzias latipes. Annot Zool Japon 45, 159–169.
Pankhurst, M.W. (2017). A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure. J Endocrinol 233, R1–R13.
Pertea, M., Kim, D., Pertea, G.M., Leek, J.T., and Salzberg, S.L. (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11, 1650–1667.
Pfennig, F., Standke, A., and Gutzeit, H.O. (2015). The role of Amh signaling in teleost fish—Multiple functions not restricted to the gonads. Gen Comp Endocrinol 223, 87–107.
Pikulkaew, S., Benato, F., Celeghin, A., Zucal, C., Skobo, T., Colombo, L., and Dalla Valle, L. (2011). The knockdown of maternal glucocorticoid receptor mRNA alters embryo development in zebrafish. Dev Dyn 240, 874–889.
Pottinger, T.G., and Pickering, A.D. (1990). The effect of cortisol administration on hepatic and plasma estradiol-binding capacity in immature female rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 80, 264–273.
Prasad, S., Tiwari, M., Pandey, A.N., Shrivastav, T.G., and Chaube, S.K. (2016). Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci 23, 36.
Ruiz-Cortes, Z.T., Kimmins, S., Monaco, L., Burns, K.H., Sassone-Corsi, P., and Murphy, B.D. (2005). Estrogen mediates phosphorylation of histone H3 in ovarian follicle and mammary epithelial tumor cells via the mitotic kinase, Aurora B. Mol Endocrinol 19, 2991–3000.
Sánchez, F., and Smitz, J. (2012). Molecular control of oogenesis. Biochim Biophys Acta 1822, 1896–1912.
Sasson, R., Tajima, K., and Amsterdam, A. (2001). Glucocorticoids protect against apoptosis induced by serum deprivation, cyclic adenosine 3′,5′-monophosphate and p53 activation in immortalized human granulosa cells: involvement of Bcl-2. Endocrinology 142, 802–811.
Sasson, R., Winder, N., Kees, S., and Amsterdam, A. (2002). Induction of apoptosis in granulosa cells by TNFα and its attenuation by glucocorticoids involve modulation of Bcl-2. Biochem Biophys Res Commun 294, 51–59.
Sasson, R., and Amsterdam, A. (2003). Pleiotropic anti-apoptotic activity of glucocorticoids in ovarian follicular cells. Biochem Pharmacol 66, 1393–1401.
Sopinka, N.M., Capelle, P.M., Semeniuk, C.A.D., and Love, O.P. (2017). Glucocorticoids in fish eggs: variation, interactions with the environment, and the potential to shape offspring fitness. Physiol Biochem Zool 90, 15–33.
Sun, J., Yan, L., Shen, W., and Meng, A. (2018). Maternal Ybx1 safeguards zebrafish oocyte maturation and maternal-to-zygotic transition by repressing global translation. Development 145, dev166587.
Suzuki, A., and Shibata, N. (2004). Developmental process of genital ducts in the medaka, Oryzias latipes. Zool Sci 21, 397–406.
Suzuki, A., Tanaka, M., Shibata, N., and Nagahama, Y. (2004). Expression of aromatase mRNA and effects of aromatase inhibitor during ovarian development in the medaka, Oryzias latipes. J Exp Zool 301A, 266–273.
Visser, J.A., Durlinger, A.L.L., Peters, I.J.J., van den Heuvel, E.R., Rose, U.M., Kramer, P., de Jong, F.H., and Themmen, A.P.N. (2007). Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology 148, 2301–2308.
Walters, K.A., Allan, C.M., and Handelsman, D.J. (2008). Androgen actions and the ovary. Biol Reprod 78, 380–389.
Wang, D.S., Kobayashi, T., Zhou, L.Y., Paul-Prasanth, B., Ijiri, S., Sakai, F., Okubo, K., Morohashi, K., and Nagahama, Y. (2007). Foxl2 upregulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol Endocrinol 21, 712–725.
Wendelaar Bonga, S.E. (1997). The stress response in fish. Physiol Rev 77, 591–625.
Whirledge, S., and Cidlowski, J.A. (2017). Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab 28, 399–415.
Wu, X., Viveiros, M.M., Eppig, J.J., Bai, Y., Fitzpatrick, S.L., and Matzuk, M.M. (2003). Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat Genet 33, 187–191.
Xu, P.F., and Meng, A.M. (2014). Maternal ractors regulate embryonic development in zebrafish (in Chinese). Sci Sin Vitae, 44, 954–964.
Yamaguchi, T., Yoshinaga, N., Yazawa, T., Gen, K., and Kitano, T. (2010). Cortisol is involved in temperature-dependent sex determination in the Japanese flounder. Endocrinology 151, 3900–3908.
Yan, L., Feng, H., Wang, F., Lu, B., Liu, X., Sun, L., and Wang, D. (2019a). Establishment of three estrogen receptors (esr1, esr2a, esr2b) knockout lines for functional study in Nile tilapia. J Steroid Biochem Mol Biol 191, 105379.
Yan, Y.L., Batzel, P., Titus, T., Sydes, J., Desvignes, T., BreMiller, R., Draper, B., and Postlethwait, J.H. (2019b). A hormone that lost its receptor: anti-Müllerian hormone (AMH) in zebrafish gonad development and sex determination. Genetics 213, 529–553.
Yuan, H.J., Li, Z.B., Zhao, X.Y., Sun, G.Y., Wang, G.L., Zhao, Y.Q., Zhang, M., and Tan, J.H. (2020). Glucocorticoids impair oocyte competence and trigger apoptosis of ovarian cells via activating the TNF-α system. Reproduction 160, 129–140.
Yuan, X.H., Yang, B.Q., Hu, Y., Fan, Y.Y., Zhang, L.X., Zhou, J.C., Wang, Y.Q., Lu, C.L., and Ma, X. (2014). Dexamethasone altered steroidogenesis and changed redox status of granulosa cells. Endocrine 47, 639–647.
Zhang, Q., Ye, D., Wang, H., Wang, Y., Hu, W., and Sun, Y. (2020). Zebrafish cyp11c1 knockout reveals the roles of 11-ketotestosterone and cortisol in sexual development and reproduction. Endocrinology 161, bqaa048.
Zhang, S.Y., Wang, J.Z., Li, J.J., Wei, D.L., Sui, H.S., Zhang, Z.H., Zhou, P., and Tan, J.H. (2011a). Maternal restraint stress diminishes the developmental potential of oocytes. Biol Reprod 84, 672–681.
Zhang, W.L., Zhou, L.Y., Senthilkumaran, B., Huang, B.F., Sudhakumari, C.C., Kobayashi, T., Nagahama, Y., and Wang, D.S. (2010). Molecular cloning of two isoforms of 11β-hydroxylase and their expressions in the Nile tilapia, Oreochromis niloticus. Gen Comp Endocrinol 165, 34–41.
Zhang, X., Min, Q., Li, M., Liu, X., Li, M., and Wang, D. (2019). Mutation of cyp19a1b results in sterile males due to efferent duct obstruction in Nile tilapia. Mol Reprod Dev 86, 1224–1235.
Zhang, X.P., Liu, F., and Wang, W. (2011b). Two-phase dynamics of p53 in the DNA damage response. Proc Natl Acad Sci USA 108, 8990–8995.
Zheng, Q., Xiao, H., Shi, H., Wang, T., Sun, L., Tao, W., Kocher, T.D., Li, M., and Wang, D. (2020). Loss of Cyp11c1 causes delayed spermatogenesis due to the absence of 11-ketotestosterone. J Endocrinol 244, 487–499.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFD0900202), the National Natural Science Foundation of China (31972778, 31861123001, 31630082 and 31872556), the Chongqing Science and Technology Commission (cstc2018jscx-mszd0380 and cstc2018jcyjAX0283), and Yunnan Science and Technology project (2018IB019). We are indebted to Thomas D. Kocher from University of Maryland, USA, for correcting the English of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xiao, H., Xu, Z., Zhu, X. et al. Cortisol safeguards oogenesis by promoting follicular cell survival. Sci. China Life Sci. 65, 1563–1577 (2022). https://doi.org/10.1007/s11427-021-2051-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-021-2051-0