Abstract
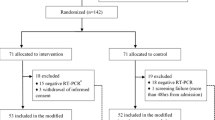
Coronavirus disease 2019 (COVID-19) is a pandemic with no specific drugs and high fatality. The most urgent need is to find effective treatments. We sought to determine whether hydroxychloroquine (HCQ) application may reduce the death risk of critically ill COVID-19 patients. In this retrospective study, we included 550 critically ill COVID-19 patients who need mechanical ventilation in Tongji Hospital, Wuhan, from February 1, 2020 to April 4, 2020. All 550 patients received comparable basic treatments including antiviral drugs and antibiotics, and 48 of them were treated with oral HCQ treatment (200 mg twice a day for 7–10 days) in addition to the basic treatments. Primary endpoint is fatality of patients, and inflammatory cytokine levels were compared between HCQ and non-hydroxychloroquine (NHCQ) treatments. We found that fatalities are 18.8% (9/48) in HCQ group, which is significantly lower than 47.4% (238/502) in the NHCQ group (P<0.001). The time of hospital stay before patient death is 15 (10–21) days and 8 (4–14) days for the HCQ and NHCQ groups, respectively (P<0.05). The levels of inflammatory cytokine IL-6 were significantly reduced from 22.2 (8.3-118.9) pg mL–1 at the beginning of the treatment to 5.2 (3.0–23.4) pg mL–1 (P<0.05) at the end of the treatment in the HCQ group but there is no change in the NHCQ group. These data demonstrate that addition of HCQ on top of the basic treatments is highly effective in reducing the fatality of critically ill patients of COVID-19 through attenuation of inflammatory cytokine storm. Therefore, HCQ should be prescribed as a part of treatment for critically ill COVID-19 patients, with possible outcome of saving lives. hydroxychloroquine, IL-6, mortalities, COVID-19
Article PDF
Similar content being viewed by others
Change history
18 June 2020
<OrderedList><ListItem><ItemNumber>1.</ItemNumber><ItemContent><Para>In the abstract, we missed a piece of information. The correct sentence should be “In this retrospective study, we included 550 critically ill COVID-19 patients who need mechanical ventilation <Emphasis Type="Bold">(63.5%) and oxygen therapy (35.6%)</Emphasis> in Tongji Hospital, Wuhan, from February 1, 2020 to April 4, 2020.”</Para></ItemContent></ListItem><ListItem><ItemNumber>2.</ItemNumber><ItemContent><Para>We mistakenly put an approval number from Tongji Hospital ethics committee in the paper (IRBID: TJ-C20200113). The correct number should be <Emphasis Type="Bold">TJ-IRB20200229</Emphasis>.</Para></ItemContent></ListItem><ListItem><ItemNumber>3.</ItemNumber><ItemContent><Para>We mistakenly filled some data in Table 1 and the correct Table 1 (the corrected data are in boldface) should be as follows:</Para></ItemContent></ListItem></OrderedList>
References
Al-Bari, M.A.A. (2015). Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother 70, 1608–1621.
Bian, X., Shi, Z., Chen, R., Cai, J., Wang, C., Xie, J., Zhao, L., Fei, X., Zhang, H., Tan, Y., et al. (2020). Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. Immunol Pathol, https://doi.org/10.21203/rs.3.rs-19346/v1.
Gautret, P., Lagier, J.C., Parola, P., Hoang, V.T., Meddeb, L., Mailhe, M., Doudier, B., Courjon, J., Giordanengo, V., Vieira, V.E., et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Ag 20, 105949.
Geleris, J., Sun, Y., Platt, J., Zucker, J., Baldwin, M., Hripcsak, G., Labella, A., Manson, D., Kubin, C., Barr, R.G., et al. (2020). Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med https://doi.org/10.1056/NEJMoa2012410.
Grein, J., Ohmagari, N., Shin, D., Diaz, G., Asperges, E., Castagna, A., Feldt, T., Green, G., Green, M.L., Lescure, F.X., et al. (2020). Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med https://doi.org/10.1056/NEJMoa2007016.
Guan, W.J., Ni, Z.Y., Hu, Y., Liang, W.H., Ou, C.Q., He, J.X., Liu, L., Shan, H., Lei, C.L., Hui, D.S.C., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382, 1708–1720.
He, Y., Xu, Y., Zhang, C., Gao, X., Dykema, K.J., Martin, K.R., Ke, J., Hudson, E.A., Khoo, S.K., Resau, J.H., et al. (2011). Identification of a lysosomal pathway that modulates glucocorticoid signaling and the inflammatory response. Sci Signal 4, ra44.
Luo, P., Liu, Y., Qiu, L., Liu, X., Liu, D., and Li, J. (2020). Tocilizumab treatment in COVID-19: a single center experience. J Med Virol https://doi.org/10.1002/jmv.25801.
Magagnoli, J., Narendran, S., Pereira, F., Cummings, T., Hardin, J., Sutton, S.S., and Ambati, J. (2020). Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. MedRxiv, https://doi.org/10.1101/2020.04.16.20065920.
Mahevas, M., Thi Tran, V., Roumier, M., Chabrol, A., Paule, R., Guillaud, C., Gallien, S., Lepeule, R., Szwebel, T.A., Lescure, X., et al. (2020). No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. MedRxiv, https://doi.org/10.1101/2020.04.10.20060699.
McChesney, E.W., Banks Jr., W.F., and Fabian, R.J. (1967). Tissue distribution of chloroquine, hydroxychloroquine, and desethylchloroquine in the rat. Toxicol Appl Pharmacol 10, 501–513.
National Health Commission of China. (2020). Chinese management guideline for COVID-19 (version 5.0). http://wwwnhcgovcn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml.
Plantone, D., and Koudriavtseva, T. (2018). Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Invest 38, 653–671.
Rainsford, K.D., Parke, A.L., Clifford-Rashotte, M., and Kean, W.F. (2015). Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 23, 231–269.
Sarzi-Puttini, P., Giorgi, V., Sirotti, S., Marotto, D., Ardizzone, S., Riz-zardini, G., Antinori, S., and Galli, M. (2020). COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 38, 337–342.
Shiratori, H., Feinweber, C., Luckhardt, S., Wallner, N., Geisslinger, G., Weigert, A., and Parnham, M.J. (2018). An in vitro test system for compounds that modulate human inflammatory macrophage polarization. Eur J Pharmacol 833, 328–338.
Snijder, E.J., van der Meer, Y., Zevenhoven-Dobbe, J., Onderwater, J.J.M., van der Meulen, J., Koerten, H.K., and Mommaas, A.M. (2006). Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. JVI 80, 5927–5940.
Yao, X., Ye, F., Zhang, M., Cui, C., Huang, B., Niu, P., Liu, X., Zhao, L., Dong, E., Song, C., et al. (2020). In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis https://doi.org/10.1093/cid/ciaa237.
Zhang, C., Wu, Z., Li, J.W., Zhao, H., and Wang, G.Q. (2020). The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Ag 29, 105954.
Acknowledgements
This work was supported in part by projects from Ministry of Science and Technology of China (2020YFC0844500), the National Natural Science Foundation of China (31130031), Emergency Project Fund of Chinese Academy of Sciences (2020YJFK0105) and Chinese Academy of Engineering and Ma Yun Foundation (2020-CMKYGG-05). We thank all our colleagues from the Department of Internal Medicine, Tongji Hospital, as well as all the medical staff fighting against COVID-19, for their tremendous efforts. We also thank Professor H. Eric Xu in Shanghai Institute of Materia Medica, Chinese Academy of Sciences for his great help in writing this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no conflict of interest. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Rights and permissions
About this article
Cite this article
Yu, B., Li, C., Chen, P. et al. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci. China Life Sci. 63, 1515–1521 (2020). https://doi.org/10.1007/s11427-020-1732-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-020-1732-2