Abstract
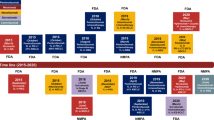
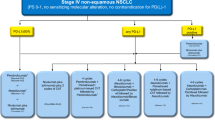
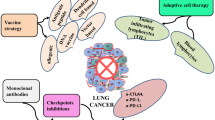
Immune checkpoint inhibitors (ICIs), especially inhibitors of the PD-1/PD-L1 axis, have significantly affected the outcomes of patients with lung cancer. Nivolumab and pembrolizumab have been approved as PD-1 blocking antibodies, whereas atezolizumab, avelumab, and durvalumab are approved as PD-L1 blocking antibodies by the United States Food and Drug Administration. However, which patient may benefit the most and how to identify patients at risk of primary or acquired resistance has not been completely defined. Meanwhile, close attention has been paid to the ongoing international and domestic clinical trials in Chinese patients with lung cancer. This review aimed to provide deep insight into the effectiveness of PD-1/PD-L1 inhibitors in patients with lung cancer, including the current settings for varied disease status, the predictive biomarkers, the resistance to ICIs, and the ongoing clinical trials in Chinese patients.
Similar content being viewed by others
References
Agata, Y., Kawasaki, A., Nishimura, H., Ishida, Y., Tsubat, T., Yagita, H., and Honjo, T. (1996). Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8, 765–772.
Akbay, E.A., Koyama, S., Carretero, J., Altabef, A., Tchaicha, J.H., Christensen, C.L., Mikse, O.R., Cherniack, A.D., Beauchamp, E.M., Pugh, T.J., et al. (2013). Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3, 1355–1363.
Anagnostou, V., Smith, K.N., Forde, P.M., Niknafs, N., Bhattacharya, R., White, J., Zhang, T., Adleff, V., Phallen, J., Wali, N., et al. (2017). Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov 7, 264–276.
Antonia, S.J., López-Martin, J.A., Bendell, J., Ott, P.A., Taylor, M., Eder, J. P., Jäger, D., Pietanza, M.C., Le, D.T., de Braud, F., et al. (2016). Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol 17, 883–895.
Antonia, S.J., Villegas, A., Daniel, D., Vicente, D., Murakami, S., Hui, R., Kurata, T., Chiappori, A., Lee, K.H., de Wit, M., et al. (2018). Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379, 2342–2350.
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D.R., Steins, M., Ready, N.E., Chow, L.Q., Vokes, E.E., Felip, E., Holgado, E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373, 1627–1639.
Brahmer, J., Reckamp, K.L., Baas, P., Crinò, L., Eberhardt, W.E.E., Poddubskaya, E., Antonia, S., Pluzanski, A., Vokes, E.E., Holgado, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373, 123–135.
Brahmer, J., Rodríguez-Abreu, D., Robinson, A., Hui, R., Csőszi, T., Fülöp, A., Gottfried, M., Peled, N., Tafreshi, A., Cuffe, S., et al. (2017). OA 17.06 updated analysis of KEYNOTE-024: Pembrolizumab vs platinum-based chemotherapy for advanced NSCLC with PD-L1 TPS ≥50%. J Thorac Oncol 12, S1793–S1794.
Carbone, D.P., Reck, M., Paz-Ares, L., Creelan, B., Horn, L., Steins, M., Felip, E., van den Heuvel, M.M., Ciuleanu, T.E., Badin, F., et al. (2017). First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 376, 2415–2426.
Casey, S.C., Tong, L., Li, Y., Do, R., Walz, S., Fitzgerald, K.N., Gouw, A. M., Baylot, V., Gütgemann, I., Eilers, M., et al. (2016). MYC regulates the antitumor immune response through CD47 and PD-L1. Science 352, 227–231.
Chen, L., Ashe, S., Brady, W.A., Hellström, I., Hellström, K.E., Ledbetter, J.A., McGowan, P., and Linsley, P.S. (1992). Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 71, 1093–1102.
Chen, L., and Flies, D.B. (2013). Molecular mechanisms of T cell costimulation and co-inhibition. Nat Rev Immunol 13, 227–242.
Chen, W., Zheng, R., Zeng, H., and Zhang, S. (2015). Epidemiology of lung cancer in China. Thorac Cancer 6, 209–215.
Chen, Y., Mu, C.Y., and Huang, J.A. (2012). Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: A 5-year-follow-up study. Tumori J 98, 751–755.
Chung, H.C., Lopez-Martin, J.A., Kao, S.C.H., Miller, W.H., Ros, W., Gao, B., Marabelle, A., Gottfried, M., Zer, A., Delord, J.P., et al. (2018). Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol 36, 8506.
Doroshow, D.B., and Herbst, R.S. (2018). Treatment of advanced non-small cell lung cancer in 2018. JAMA Oncol 4, 569–570.
Fehrenbacher, L., Spira, A., Ballinger, M., Kowanetz, M., Vansteenkiste, J., Mazieres, J., Park, K., Smith, D., Artal-Cortes, A., Lewanski, C., et al. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846.
Felip, E., Hellmann, M.D., Hui, R., Carcereny, E., Leighl, N.B., Ahn, M.J., Eder, J.P., Balmanoukian, A.S., Aggarwal, C., Horn, L., et al. (2018). 4-year overall survival for patients with advanced NSCLC treated with pembrolizumab: Results from KEYNOTE-001. J Clin Oncol 36, 9030.
Gainor, J.F., Shaw, A.T., Sequist, L.V., Fu, X., Azzoli, C.G., Piotrowska, Z., Huynh, T.G., Zhao, L., Fulton, L., Schultz, K.R., et al. (2016). EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res 22, 4585–4593.
Gandara, D.R., Paul, S.M., Kowanetz, M., Schleifman, E., Zou, W., Li, Y., Rittmeyer, A., Fehrenbacher, L., Otto, G., Malboeuf, C., et al. (2018). Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 24, 1441–1448.
Gandhi, L., Rodríguez-Abreu, D., Gadgeel, S., Esteban, E., Felip, E., De Angelis, F., Domine, M., Clingan, P., Hochmair, M.J., Powell, S.F., et al. (2018). Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378, 2078–2092.
Garassino, M.C., Cho, B.C., Kim, J.H., Mazières, J., Vansteenkiste, J., Lena, H., Corral Jaime, J., Gray, J.E., Powderly, J., Chouaid, C., et al. (2018). Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet Oncol 19, 521–536.
Garon, E.B., Rizvi, N.A., Hui, R., Leighl, N., Balmanoukian, A.S., Eder, J. P., Patnaik, A., Aggarwal, C., Gubens, M., Horn, L., et al. (2015). Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372, 2018–2028.
Garon, E.B., Hellmann, M.D., Rizvi, N.A., Carcereny, E., Leighl, N.B., Ahn, M.J., Eder, J.P., Balmanoukian, A.S., Aggarwal, C., Horn, L., et al. (2019). Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol 37, 2518–2527.
Gentzler, R.D., Langer, C.J., Borghaei, H., Gadgeel, S.M., Papadimitrakopoulou, V., Patnaik, A., Powell, S.F., Martins, R.G., Stevenson, J., Jalal, S.I., et al. (2018). 24-month overall survival from KEYNOTE-021 cohort G: Pemetrexed-carboplatin plus pembrolizumab as first-line therapy for advanced nonsquamous NSCLC. J Clin Oncol 36, 9026.
Gettinger, S., Choi, J., Hastings, K., Truini, A., Datar, I., Sowell, R., Wurtz, A., Dong, W., Cai, G., Melnick, M.A., et al. (2017). Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov 7, 1420–1435.
Gettinger, S., Horn, L., Jackman, D., Spigel, D., Antonia, S., Hellmann, M., Powderly, J., Heist, R., Sequist, L.V., Smith, D.C., et al. (2018). Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: Results from the CA209-003 study. J Clin Oncol 36, 1675–1684.
Gopalakrishnan, V., Spencer, C.N., Nezi, L., Reuben, A., Andrews, M.C., Karpinets, T.V., Prieto, P.A., Vicente, D., Hoffman, K., Wei, S.C., et al. (2018). Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103.
Harlin, H., Meng, Y., Peterson, A.C., Zha, Y., Tretiakova, M., Slingluff, C., McKee, M., and Gajewski, T.F. (2009). Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 69, 3077–3085.
Havel, J.J., Chowell, D., and Chan, T.A. (2019). The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 19, 133–150.
Hellmann, M.D., Ciuleanu, T.E., Pluzanski, A., Lee, J.S., Otterson, G.A., Audigier-Valette, C., Minenza, E., Linardou, H., Burgers, S., Salman, P., et al. (2018a). Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378, 2093–2104.
Hellmann, M.D., Nathanson, T., Rizvi, H., Creelan, B.C., Sanchez-Vega, F., Ahuja, A., Ni, A., Novik, J.B., Mangarin, L.M.B., Abu-Akeel, M., et al. (2018b). Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell 33, 843–852.e4.
Herbst, R.S., Soria, J.C., Kowanetz, M., Fine, G.D., Hamid, O., Gordon, M. S., Sosman, J.A., McDermott, D.F., Powderly, J.D., Gettinger, S.N., et al. (2014). Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567.
Herbst, R.S., Gandara, D.R., Hirsch, F.R., Redman, M.W., LeBlanc, M., Mack, P.C., Schwartz, L.H., Vokes, E., Ramalingam, S.S., Bradley, J.D., et al. (2015). Lung master protocol (lung-MAP)—A biomarker-driven protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin Cancer Res 21, 1514–1524.
Herbst, R.S., and Sznol, M. (2016). Diminished but not dead: Chemotherapy for the treatment of NSCLC. Lancet Oncol 17, 1464–1465.
Hersom, M., and Jørgensen, J.T. (2018). Companion and complementary diagnostics-focus on PD-L1 expression assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Ther Drug Monit 40, 9–16.
Hirsch, F.R., McElhinny, A., Stanforth, D., Ranger-Moore, J., Jansson, M., Kulangara, K., Richardson, W., Towne, P., Hanks, D., Vennapusa, B., et al. (2017). PD-L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 12, 208–222.
Horn, L., Reck, M., Mok, T., Johnson, M.L., Tang, X., Lam, S., Waterkamp, D., Lopez-Chavez, A., Sandler, A., Giacconne, G., et al. (2016). PS01.57: IMpower133: A phase I/III study of 1L atezolizumab with carboplatin and etoposide in patients with extensive-stage SCLC. J Thorac Oncol 11, S305–S306.
Horn, L., Spigel, D.R., Vokes, E.E., Holgado, E., Ready, N., Steins, M., Poddubskaya, E., Borghaei, H., Felip, E., Paz-Ares, L., et al. (2017). Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: Two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 35, 3924–3933.
Hugo, W., Shi, H., Sun, L., Piva, M., Song, C., Kong, X., Moriceau, G., Hong, A., Dahlman, K.B., Johnson, D.B., et al. (2015). Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell 162, 1271–1285.
Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T., and Minato, N. (2002). Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99, 12293–12297.
Jiang, L., Su, X., Zhang, T., Yin, X., Zhang, M., Fu, H., Han, H., Sun, Y., Dong, L., Qian, J., et al. (2017). PD-L1 expression and its relationship with oncogenic drivers in non-small cell lung cancer (NSCLC). Oncotarget 8, 26845–26857.
Jin, Y., Dong, H., Xia, L., Yang, Y., Zhu, Y., Shen, Y., Zheng, H., Yao, C., Wang, Y., and Lu, S. (2019). The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in chinese patients with NSCLC. J Thorac Oncol 14, 1378–1389.
Kaderbhaï, C., Tharin, Z., and Ghiringhelli, F. (2019). The role of molecular profiling to predict the response to immune checkpoint inhibitors in lung cancer. Cancers 11, 201.
Karwacz, K., Bricogne, C., MacDonald, D., Arce, F., Bennett, C.L., Collins, M., and Escors, D. (2011). PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med 3, 581–592.
Keir, M.E., Liang, S.C., Guleria, I., Latchman, Y.E., Qipo, A., Albacker, L. A., Koulmanda, M., Freeman, G.J., Sayegh, M.H., and Sharpe, A.H. (2006). Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203, 883–895.
Kelderman, S., Schumacher, T.N.M., and Haanen, J.B.A.G. (2014). Acquired and intrinsic resistance in cancer immunotherapy. Mol Oncol 8, 1132–1139.
Koyama, S., Akbay, E.A., Li, Y.Y., Aref, A.R., Skoulidis, F., Herter-Sprie, G.S., Buczkowski, K.A., Liu, Y., Awad, M.M., Denning, W.L., et al. (2016a). STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res 76, 999–1008.
Koyama, S., Akbay, E.A., Li, Y.Y., Herter-Sprie, G.S., Buczkowski, K.A., Richards, W.G., Gandhi, L., Redig, A.J., Rodig, S.J., Asahina, H., et al. (2016b). Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 7, 10501.
Lee, C.K., Man, J., Lord, S., Cooper, W., Links, M., Gebski, V., Herbst, R. S., Gralla, R.J., Mok, T., and Yang, J.C.H. (2018). Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma. JAMA Oncol 4, 210–216.
Mok, T.S.K., Wu, Y.L., Kudaba, I., Kowalski, D.M., Cho, B.C., Turna, H. Z., Castro Jr, G., Srimuninnimit, V., Laktionov, K.K., Bondarenko, I., et al. (2019). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393, 1819–1830.
Mokhles, S., Nuyttens, J.J., Maat, A.P.W.M., Birim, Ö., Aerts, J.G.J.V., Bogers, A.J.J.C., and Takkenberg, J.J.M. (2015). Survival and treatment of non-small cell lung cancer stage I-II treated surgically or with stereotactic body radiotherapy: Patient and tumor-specific factors affect the prognosis. Ann Surg Oncol 22, 316–323.
Papadimitrakopoulou, V., Cobo, M., Bordoni, R., Dubray-Longeras, P., Szalai, Z., Ursol, G., Novello, S., Orlandi, F., Ball, S., Goldschmidt Jr., J., et al. (2018). OA05.07 IMpower132: PFS and Safety results with 1L atezolizumab+carboplatin/cisplatin+pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol 13, S332–S333.
Pardoll, D.M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12, 252–264.
Parsa, A.T., Waldron, J.S., Panner, A., Crane, C.A., Parney, I.F., Barry, J.J., Cachola, K.E., Murray, J.C., Tihan, T., Jensen, M.C., et al. (2007). Loss of tumor suppressor PTEN function increases B7–H1 expression and immunoresistance in glioma. Nat Med 13, 84–88.
Paz-Ares, L., Luft, A., Vicente, D., Tafreshi, A., Gümüş., M., Mazières, J., Hermes, B., Çay Şenler, F., Csőszi, T., Fülöp, A., et al. (2018). Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379, 2040–2051.
Peng, W., Chen, J.Q., Liu, C., Malu, S., Creasy, C., Tetzlaff, M.T., Xu, C., McKenzie, J.A., Zhang, C., Liang, X., et al. (2016). Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov 6, 202–216.
Rajan, A., Gulley, J.L., Spigel, D.R., Iannotti, N., Chandler, J.C., Wong, D. J.L., Leach, J.W., Edenfield, W.J., Wang, D., Redfern, C.H., et al. (2018). Avelumab (anti-PD-L1) in patients with platinum-treated advanced NSCLC: 2.5-year follow-up from the JAVELIN Solid Tumor trial. J Clin Oncol 36, 9090.
Reck, M., Mok, T.S.K., Nishio, M., Jotte, R.M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., Nogami, N., Rodríguez-Abreu, D., Moro-Sibilot, D., et al. (2019). Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 7, 387–401.
Riaz, N., Havel, J.J., Makarov, V., Desrichard, A., Urba, W.J., Sims, J.S., Hodi, F.S., Martín-Algarra, S., Mandal, R., Sharfman, W.H., et al. (2017). Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171, 934–949.e16.
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., von Pawel, J., Gadgeel, S.M., Hida, T., Kowalski, D.M., Dols, M.C., et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265.
Rizvi, H., Sanchez-Vega, F., La, K., Chatila, W., Jonsson, P., Halpenny, D., Plodkowski, A., Long, N., Sauter, J.L., Rekhtman, N., et al. (2018). Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clini Oncol 36, 633–641.
Routy, B., Le Chatelier, E., Derosa, L., Duong, C.P.M., Alou, M.T., Daillère, R., Fluckiger, A., Messaoudene, M., Rauber, C., Roberti, M.P., et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97.
Seban, R.D., Mezquita, L., Berenbaum, A., Dercle, L., Botticella, A., Le Pechoux, C., Caramella, C., Deutsch, E., Grimaldi, S., Adam, J., et al. (2019). Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur J Nucl Med Mol Imag doi: https://doi.org/10.1007/s00259-019-04615-x.
Skoulidis, F., Goldberg, M.E., Greenawalt, D.M., Hellmann, M.D., Awad, M.M., Gainor, J.F., Schrock, A.B., Hartmaier, R.J., Trabucco, S.E., Gay, L., et al. (2018). STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 8, 822–835.
Socinski, M.A., Jotte, R.M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., Nogami, N., Rodríguez-Abreu, D., Moro-Sibilot, D., Thomas, C.A., Barlesi, F., et al. (2018). Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378, 2288–2301.
Spranger, S., Bao, R., and Gajewski, T.F. (2015). Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235.
Squibb, B. M. (2019). Bristol-Myers Squibb provides update on the ongoing regulatory review of Opdivo plus low-dose Yervoy in first-line lung cancer patients with tumor mutational burden≥10 mut/Mb. 2018.
Tang, H., Liang, Y., Anders, R.A., Taube, J.M., Qiu, X., Mulgaonkar, A., Liu, X., Harrington, S.M., Guo, J., Xin, Y., et al. (2018). PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest 128, 580–588.
Taube, J.M., Anders, R.A., Young, G.D., Xu, H., Sharma, R., McMiller, T. L., Chen, S., Klein, A.P., Pardoll, D.M., Topalian, S.L., et al. (2012). Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4, 127ra37.
Taube, J.M., Young, G.D., McMiller, T.L., Chen, S., Salas, J.T., Pritchard, T.S., Xu, H., Meeker, A.K., Fan, J., Cheadle, C., et al. (2015). Differential Expression of immune-regulatory genes associated with PD-L1 display in melanoma: Implications for PD-1 pathway blockade. Clin Cancer Res 21, 3969–3976.
Tsushima, F., Yao, S., Shin, T., Flies, A., Flies, S., Xu, H., Tamada, K., Pardoll, D.M., and Chen, L. (2007). Interaction between B7–H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood 110, 180–185.
Woo, S.R., Corrales, L., and Gajewski, T.F. (2015). Innate immune recognition of cancer. Annu Rev Immunol 33, 445–474.
Yarchoan, M., Hopkins, A., and Jaffee, E.M. (2017). Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377, 2500–2501.
Yue, C., Shen, S., Deng, J., Priceman, S.J., Li, W., Huang, A., and Yu, H. (2015). STAT3 in CD8+ T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 axis. Cancer Immunol Res 3, 864–870.
Zhang, W.Q., Zhao, S.K., Luo, J.W., Dong, X.P., Hao, Y.T., Li, H., Shan, L., Zhou, Y., Shi, H.B., Zhang, Z.Y., et al. (2018). Alterations of fecal bacterial communities in patients with lung cancer. Am J Transl Res 10, 3171–3185.
Zhang, Y., and Chen, L. (2016). Classification of advanced human cancers based on tumor immunity in the microenvironment (TIME) for cancer immunotherapy. JAMA Oncol 2, 1403–1404.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFC1303300), the National Natural Science Foundation of China (81672272), the Shanghai Municipal Science and Technology Commission Research Project (17431906103), the Shanghai Chest Hospital Project of Collaborative Innovation (YJXT20190105), and the Clinical Research Plan of Shanghai Hospital Development Center (SHDC) (16CR3005A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Compliance and ethics
The author(s) declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, Y., Yu, Y. & Lu, S. Effectiveness of PD-1/PD-L1 inhibitors in the treatment of lung cancer: Brightness and challenge. Sci. China Life Sci. 63, 1499–1514 (2020). https://doi.org/10.1007/s11427-019-1622-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-019-1622-5