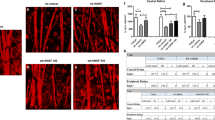
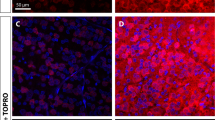
Abstract
Optic neuropathies lead to blindness; the common pathology is the degeneration of axons of the retinal ganglion cells. In this study, we used a rat model of retinal ischemia-reperfusion and a one-time intravitreal brain-derived neurotrophic factor (BDNF) injection; then we examined axon transportation function, continuity, physical presence of axons in different part of the optic nerve, and the expression level of proteins involved in axon transportation. We found that in the disease model, axon transportation was the most severely affected, followed by axon continuity, then the number of axons in the distal and proximal optic nerve. BDNF treatment relieved all reductions and significantly restored function. The molecular changes were more minor, probably due to massive gliosis of the optic nerve, so interpretation of protein expression data should be done with some caution. The process in this acute model resembles a fast-forward of changes in the chronic model of glaucoma. Therefore, impairment in axon transportation appears to be a common early process underlying different optic neuropathies. This research on effective intervention can be used to develop interventions for all optic neuropathies targeting axon transportation.
Similar content being viewed by others
References
Alexander, C., Votruba, M., Pesch, U.E.A., Thiselton, D.L., Mayer, S., Moore, A., Rodriguez, M., Kellner, U., Leo-Kottler, B., Auburger, G., et al. (2000). OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26, 211–215.
Biousse, V., and Newman, N. (2014). Retinal and optic nerve ischemia. Continuum (Minneap Minn) 20, 838–856.
Buckingham, B.P., Inman, D.M., Lambert, W., Oglesby, E., Calkins, D.J., Steele, M.R., Vetter, M.L., Marsh-Armstrong, N., and Horner, P.J. (2008). Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci 28, 2735–2744.
Cavanagh, J. (1979). The ‘dying back’ process. A common denominator in many naturally occurring and toxic neuropathies. Arch Pathol Lab Med 103, 659–664.
Coleman, M. (2005). Axon degeneration mechanisms: Commonality amid diversity. Nat Rev Neurosci 6, 889–898.
Crish, S.D., Sappington, R.M., Inman, D.M., Horner, P.J., and Calkins, D.J. (2010). Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci USA 107, 5196–5201.
Crish, S.D., Dapper, J.D., MacNamee, S.E., Balaram, P., Sidorova, T.N., Lambert, W.S., and Calkins, D.J. (2013). Failure of axonal transport induces a spatially coincident increase in astrocyte BDNF prior to synapse loss in a central target. Neuroscience 229, 55–70.
Cueva Vargas, J.L., Belforte, N., and Di Polo, A. (2016). The glial cell modulator ibudilast attenuates neuroinflammation and enhances retinal ganglion cell viability in glaucoma through protein kinase A signaling. Neurobiol Dis 93, 156–171.
Dai, C., Khaw, P.T., Yin, Z.Q., Li, D., Raisman, G., and Li, Y. (2012). Structural basis of glaucoma: The fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia 60, 13–28.
Delettre, C., Lenaers, G., Griffoin, J.M., Gigarel, N., Lorenzo, C., Belenguer, P., Pelloquin, L., Grosgeorge, J., Turc-Carel, C., Perret, E., et al. (2000). Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26, 207–210.
Gellermann, L.W. (1933). Chance orders of alternating stimuli in visual discrimination experiments. Pedagog Semin J Genet Psychol 42, 206–208.
Hirokawa, N., and Noda, Y. (2008). Intracellular transport and kinesin superfamily proteins, KIFs: Structure, function, and dynamics. Physiol Rev 88, 1089–1118.
Hoyt, C.S. (1980). Autosomal dominant optic atrophy. Ophthalmology 87, 245–251.
Huang, L., Chen, Y., Lin, Y., Tam, P.O.S., Cheng, Y., Shi, Y., Gong, B., Lu, F., Yang, J., Wang, H., et al. (2019). Genome-wide analysis identified 17 new loci influencing intraocular pressure in Chinese population. Sci China Life Sci 62, 153–164.
Hughes, W.F. (1991). Quantitation of ischemic damage in the rat retina. Exp Eye Res 53, 573–582.
Huoponen, K., Vilkki, J., Aula, P., Nikoskelainen, E. K., Savontaus, M. (1991). A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet 48, 1147–1153.
Johns, D.R., Neufeld, M.J., and Park, R.D. (1992). An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem Biophys Res Commun 187, 1551–1557.
Kuribayashi, J., Kitaoka, Y., Munemasa, Y., and Ueno, S. (2010). Kinesin-1 and degenerative changes in optic nerve axons in NMDA-induced neurotoxicity. Brain Res 1362, 133–140.
Liu, L., Li, X., Killer, H.E., Cao, K., Li, J., and Wang, N. (2019). Changes in retinal and choroidal morphology after cerebrospinal fluid pressure reduction: A Beijing iCOP study. Sci China Life Sci 62, 268–271.
Luan, L., Ren, C., Wang, W., Nan, Y., Gao, J., and Pu, M. (2018). Morphological properties of medial amygdala-projecting retinal ganglion cells in the Mongolian gerbil. Sci China Life Sci 61, 644–650.
McGill, T.J., Douglas, R.M., Lund, R.D., and Prusky, G.T. (2004). Quantification of spatial vision in the Royal College of Surgeons rat. Invest Ophthalmol Vis Sci 45, 932.
Okada, Y., Yamazaki, H., Sekine-Aizawa, Y., and Hirokawa, N. (1995). The neuron-specific kinesin superfamily protein KIF1A is a uniqye monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81, 769–780.
Paschal, B.M., Shpetner, H.S., and Vallee, R.B. (1987). MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol 105, 1273–1282.
Patel, H.R., and Margo, C.E. (2017). Pathology of ischemic optic neuropathy. Arch Pathol Lab Med 141, 162–166.
Prusky, G.T., West, P.W.R., and Douglas, R.M. (2000). Behavioral assessment of visual acuity in mice and rats. Vision Res 40, 2201–2209.
Qiao, L., Zhang, X., Jan, C., Li, X., Li, M., and Wang, H. (2019). Macular retinal thickness and flow density change by optical coherence tomography angiography after posterior scleral reinforcement. Sci China Life Sci 62, 930–936.
Quigley, H.A., Addicks, E.M., and Green, W.R. (1982). Optic nerve damage in human glaucoma. Arch Ophthalmol 100, 135.
Reichstein, D., Ren, L., Filippopoulos, T., Mittag, T., and Danias, J. (2007). Apoptotic retinal ganglion cell death in the DBA/2 mouse model of glaucoma. Exp Eye Res 84, 13–21.
Ren, R., Li, Y., Liu, Z., Liu, K., and He, S. (2012). Long-term rescue of rat retinal ganglion cells and visual function by AAV-mediated BDNF expression after acute elevation of intraocular pressure. Invest Ophthalmol Vis Sci 53, 1003–1011.
Sadun, A. A., Win, P. H., Ross-Cisneros, F., Walker, S., Carelli, V. (2000). Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 98, 223–235.
Schnapp, B.J., and Reese, T.S. (1989). Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci USA 86, 1548–1552.
Sun, L., Han, X., and He, S. (2011). Direction-selective circuitry in rat retina develops independently of GABAergic, cholinergic and action potential activity. PLoS ONE 6, e19477.
Vale, R.D., Reese, T.S., and Sheetz, M.P. (1985a). Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42, 39–50.
Vale, R.D., Schnapp, B.J., Mitchison, T., Steuer, E., Reese, T.S., and Sheetz, M.P. (1985b). Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell 43, 623–632.
Vidal-Sanz, M., Villegas-Pérez, M.P., Bray, G.M., and Aguayo, A.J. (1988). Persistent retrograde labeling of adult rat retinal ganglion cells with the carbocyanine dye diI. Exp Neurol 102, 92–101.
Votruba, M., Fitzke, F.W., Holder, G.E., Carter, A., Bhattacharya, S.S., and Moore, A.T. (1998). Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Arch Ophthalmol 116, 351–358.
Wallace, D.C., Singh, G., Lott, M.T., Hodge, J.A., Schurr, T.G., Lezza, A. M., Elsas, L.J., and Nikoskelainen, E.K. (1988). Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 242, 1427–1430.
Wang, W., Nan, Y., Pan, Z.H., and Pu, M. (2017). Morphological evaluation of retinal ganglion cells expressing the L132C/T159C ChR2 mutant transgene in young adult cynomolgus monkeys. Sci China Life Sci 60, 1157–1167.
Wang, X., Shen, K., Lu, F., and He, S. (2019). Long-lasting impairments in rodent oxygen-induced retinopathy measured by retinal vessel density and visual function. Sci China Life Sci 62, 681–690.
Weinreb, R.N., Aung, T., and Medeiros, F.A. (2014). The pathophysiology and treatment of glaucoma: A review. JAMA 311, 1901–1911.
Whitmore, A.V., Libby, R.T., and John, S.W.M. (2005). Glaucoma: Thinking in new ways—A role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retinal Eye Res 24, 639–662.
Xia, H., Hu, Q., Li, L., Tang, X., Zou, J., Huang, L., and Li, X. (2019). Protective effects of autophagy against blue light-induced retinal degeneration in aged mice. Sci China Life Sci 62, 244–256.
Xin, C., Tian, N., Li, M., Wang, H., and Wang, N. (2018). Mechanism of the reconstruction of aqueous outflow drainage. Sci China Life Sci 61, 534–540.
Yohannan, J., and Boland, M.V. (2017). The evolving role of the relationship between optic nerve structure and function in glaucoma. Ophthalmology 124, S66–S70.
Zhang, J., Jia, H., Wang, J., Xiong, Y., Li, J., Li, X., Zhao, J., Zhang, X., You, Q., Zhu, G., et al. (2019). A novel deletion mutation, c.1296delT in the BCOR gene, is associated with oculo-facio-cardio-dental syndrome. Sci China Life Sci 62, 119–125.
Zhang, X., Yuan, Q., and Gao, X. (2018). Assessment of the MT1-MMP expression level of different cell lines by the naked eye. Sci China Life Sci 61, 492–500.
Zhou, Y., Xiao, C., and Pu, M. (2017). High glucose levels impact visual response properties of retinal ganglion cells in C57 mice—An in vitro physiological study. Sci China Life Sci 60, 1428–1435.
Acknowledgements
This work was supported by The Key Project of National Natural Science Foundation of China (31030036) to Shanghai. We would like to thank Ms. Jiaying Ju for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Compliance and ethics
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest, in the subject matter or materials discussed in this manuscript. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Du, R., Wang, X. & He, S. BDNF improves axon transportation and rescues visual function in a rodent model of acute elevation of intraocular pressure. Sci. China Life Sci. 63, 1337–1346 (2020). https://doi.org/10.1007/s11427-019-1567-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-019-1567-0