Abstract
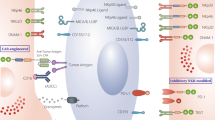
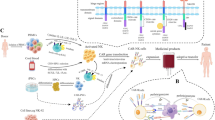
Introducing chimeric antigen receptor into immune cells against malignancies has contributed to a revolutionary innovation in cancer immunotherapy. As an important type of adaptive immune cells, T cells first caught researchers’ attention and became great success in chimeric antigen receptor-based immunotherapy. However, engineered T cells seem to hit their bottleneck when resistance of cancerous cells, less encouraging responses in solid tumors and unwanted toxicities to the host remain to be solved. Meanwhile, innate immune cells get to join the race. Representatives such as natural killer cells, natural killer T cells, γδT cells and macrophages also prove to be well redirected with chimeric antigen receptors. Compared to chimeric antigen receptor engineered T cells, these engineered innate immune cells may possess multiple targeting and killing mechanisms, have the potential to crack the barrier of solid tumors and have less side effects in the host. Besides, possible universal access to cell resources and improvements in expansion and transduction techniques make these cells promising candidates with huge potential in translational medicine. Therefore, innate immune cells claim a brand-new dimension and are likely to supplement T cells greatly in the field of chimeric antigen receptor-based immunotherapy.
Similar content being viewed by others
References
Alkins, R., Burgess, A., Ganguly, M., Francia, G., Kerbel, R., Wels, W.S., and Hynynen, K. (2013). Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res 73, 1892–1899.
Alvey, C.M., Spinler, K.R., Irianto, J., Pfeifer, C.R., Hayes, B., Xia, Y., Cho, S., Dingal, P.C.P.D., Hsu, J., Smith, L., et al. (2017). SIRPAinhibited, marrow-derived macrophages engorge, accumulate, and differentiate in antibody-targeted regression of solid tumors. Curr Biol 27, 2065–2077.e6.
Bassiri, H., Das, R., Guan, P., Barrett, D.M., Brennan, P.J., Banerjee, P.P., Wiener, S.J., Orange, J.S., Brenner, M.B., Grupp, S.A., et al. (2014). iNKT cell cytotoxic responses control T-lymphoma growth in vitro and in vivo. Cancer Immunol Res 2, 59–69.
Bendelac, A. (1995). CD1: presenting unusual antigens to unusual T lymphocytes. Science 269, 185–186.
Brennan, P.J., Brigl, M., and Brenner, M.B. (2013). Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13, 101–117.
Capsomidis, A., Benthall, G., Van Acker, H.H., Fisher, J., Kramer, A.M., Abeln, Z., Majani, Y., Gileadi, T., Wallace, R., Gustafsson, K., et al. (2018). Chimeric antigen receptor-engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol Ther 26, 354–365.
Cardell, S., Tangri, S., Chan, S., Kronenberg, M., Benoist, C., and Mathis, D. (1995). CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med 182, 993–1004.
Caruana, I., Savoldo, B., Hoyos, V., Weber, G., Liu, H., Kim, E.S., Ittmann, M.M., Marchetti, D., and Dotti, G. (2015). Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med 21, 524–529.
Chen, K.H., Wada, M., Pinz, K.G., Liu, H., Lin, K.W., Jares, A., Firor, A. E., Shuai, X., Salman, H., Golightly, M., et al. (2017). Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia 31, 2151–2160.
D'Asaro, M., La Mendola, C., Di Liberto, D., Orlando, V., Todaro, M., Spina, M., Guggino, G., Meraviglia, S., Caccamo, N., Messina, A., et al. (2010). V 9V 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol 184, 3260–3268.
Davies, D.M., and Maher, J. (2010). Adoptive T-cell immunotherapy of cancer using chimeric antigen receptor-grafted T cells. Arch Immunol Ther Exp 58, 165–178.
Deniger, D.C., Switzer, K., Mi, T., Maiti, S., Hurton, L., Singh, H., Huls, H., Olivares, S., Lee, D.A., Champlin, R.E., et al. (2013). Bispecific Tcells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther 21, 638–647.
Du, S.H., Li, Z., Chen, C., Tan, W.K., Chi, Z., Kwang, T.W., Xu, X.H., and Wang, S. (2016). Co-expansion of cytokine-induced killer cells and Vγ9Vδ2 T cells for CAR T-cell therapy. PLoS ONE 11, e0161820.
Esser, R., Müller, T., Stefes, D., Kloess, S., Seidel, D., Gillies, S.D., Aperlo-Iffland, C., Huston, J.S., Uherek, C., Schönfeld, K., et al. (2012). NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cellular Mol Med 16, 569–581.
Gholamin, S., Mitra, S.S., Feroze, A.H., Liu, J., Kahn, S.A., Zhang, M., Esparza, R., Richard, C., Ramaswamy, V., Remke, M., et al. (2017). Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 9, eaaf2968.
Harrer, D.C., Dörrie, J., and Schaft, N. (2018). Chimeric antigen receptors in different cell types: new vehicles join the race. Human Gene Ther 29, 547–558.
Heczey, A., Liu, D., Courtney, A., Marinova E., Wei, J., Tian, G., Yvan, E., Hicks J., Dotti, G., and Metelitsa L. (2013). NKT cells as a novel platform for cancer immunotherapy with chimeric antigen receptors. J Immunol 190 (1 Supplement) 2038.
Heczey, A., Liu, D., Tian, G., Courtney, A.N., Wei, J., Marinova, E., Gao, X., Guo, L., Yvon, E., Hicks, J., et al. (2014). Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 124, 2824–2833.
Jamal-Hanjani, M., Quezada, S.A., Larkin, J., and Swanton, C. (2015). Translational implications of tumor heterogeneity. Clinical Cancer Res 21, 1258–1266.
Jiang, L., and Wang, W. (2018). Genetically modified immune cells for cancer immunotherapy. Sci China Life Sci 61, 1277–1279.
Klingemann, H. (2014). Are natural killer cells superior CAR drivers? Oncoimmunology 3, e28147.
Li, J., Li, W., Huang, K., Zhang, Y., Kupfer, G., and Zhao, Q. (2018a). Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol 11, 22.
Li, Y., Hermanson, D.L., Moriarity, B.S., and Kaufman, D.S. (2018b). Human iPSC-derived natural killer cells engineered with chimeric antigen Receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192.e5.
Li, Y., Yin, J., Li, T., Huang, S., Yan, H., Leavenworth, J.M., and Wang, X. (2015). NK cell-based cancer immunotherapy: from basic biology to clinical application. Sci China Life Sci 58, 1233–1245.
Lin, C., and Zhang, J. (2018). Reformation in chimeric antigen receptor based cancer immunotherapy: Redirecting natural killer cell. BioChim Biophysica Acta (BBA)- Rev Cancer 1869, 200–215.
Liu, D., Tian, S., Zhang, K., Xiong, W., Lubaki, N.M., Chen, Z., and Han, W. (2017). Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV. Protein Cell 8, 861–877.
Liu, E., Tong, Y., Dotti, G., Shaim, H., Savoldo, B., Mukherjee, M., Orange, J., Wan, X., Lu, X., Reynolds, A., et al. (2018). Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531.
Metelitsa, L.S., Wu, H.W., Wang, H., Yang, Y., Warsi, Z., Asgharzadeh, S., Groshen, S., Wilson, S.B., and Seeger, R.C. (2004). Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med 199, 1213–1221.
Morrissey, M.A., Williamson, A.P., Steinbach, A.M., Roberts, E.W., Kern, N., Headley, M.B., and Vale, R.D. (2018). Chimeric antigen receptors that trigger phagocytosis. eLife 7, e36688.
Müller, N., Michen, S., Tietze, S., Töpfer, K., Schulte, A., Lamszus, K., Schmitz, M., Schackert, G., Pastan, I., and Temme, A. (2015). Engineering NK cells modified with an EGFRvIII-specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF-1α-secreting glioblastoma. J Immunother 38, 197–210.
Nair, S., and Dhodapkar, M.V. (2017). Natural killer T cells in cancer immunotherapy. Front Immunol 8, 1178.
Ngai, H., Tian, G., Courtney, A.N., Ravari, S.B., Guo, L., Liu, B., Jin, J., Shen, E.T., Di Pierro, E.J., and Metelitsa, L.S. (2018). IL-21 selectively protects CD62L+ NKT Cells and enhances their effector functions for adoptive immunotherapy. J Immunol 201, 2141–2153.
O’Neill, K., and Weber, S. (2017). Macrophage car (moto-car) in imunotherapy. US Patent, 20170166657A1.
Oberschmidt, O., Kloess, S., and Koehl, U. (2017). Redirected primary human chimeric antigen receptor natural killer cells as an “off-the-shelf immunotherapy” for improvement in cancer treatment. Front Immunol 8, 654.
Vantourout, P., and Hayday, A. (2013). Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 13, 88–100.
Pillai, A.B., George, T.I., Dutt, S., Teo, P., and Strober, S. (2007). Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol 178, 6242–6251.
Qian, B.Z., and Pollard, J.W. (2010). Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51.
Quail, D.F., and Joyce, J.A. (2013). Microenvironmental regulation of tumor progression and metastasis. Nat Med 19, 1423–1437.
Salio, M., Silk, J.D., Yvonne Jones, E., and Cerundolo, V. (2014). Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol 32, 323–366.
Shimasaki, N., Coustan-Smith, E., Kamiya, T., and Campana, D. (2016). Expanded and armed natural killer cells for cancer treatment. Cytotherapy 18, 1422–1434.
Song, L., Asgharzadeh, S., Salo, J., Engell, K., Wu, H., Sposto, R., Ara, T., Silverman, A.M., DeClerck, Y.A., Seeger, R.C., et al. (2009). Vα24- invariant NKT cells mediate antitumor activity via killing of tumorassociated macrophages. J Clin Invest 119, 1524–1536.
Straetemans, T., Kierkels, G.J.J., Doorn, R., Jansen, K., Heijhuurs, S., Dos Santos, J.M., van Muyden, A.D.D., Vie, H., Clemenceau, B., Raymakers, R., et al. (2018). GMP-grade manufacturing of T Cells engineered to express a defined γδTCR. Front Immunol 9, 1062.
Tao, D., Mehal, W.Z., and Crispe, I.N. (1998). IL-18 Augments perforindependent cytotoxicity of liver NK-T Cells. J Immunol 161, 2217–2222.
Terabe, M., Swann, J., Ambrosino, E., Sinha, P., Takaku, S., Hayakawa, Y., Godfrey, D.I., Ostrand-Rosenberg, S., Smyth, M.J., and Berzofsky, J.A. (2005). A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med 202, 1627–1633.
Ti, D., Niu, Y., Wu, Z., Fu, X., and Han, W. (2018). Genetic engineering of T cells with chimeric antigen receptors for hematological malignancy immunotherapy. Sci China Life Sci 61, 1320–1332.
Tian, G., Courtney, A.N., Jena, B., Heczey, A., Liu, D., Marinova, E., Guo, L., Xu, X., Torikai, H., Mo, Q., et al. (2016). CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clinical Investigation 126, 2341–2355.
Vivier, E., and Anfossi, N. (2004). Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat Rev Immunol 4, 190–198.
Vivier, E., Tomasello, E., Baratin, M., Walzer, T., and Ugolini, S. (2008). Functions of natural killer cells. Nat Immunol 9, 503–510.
Wei, J., and Han, W. (2017). CART trials are going ahead. Sci China Life Sci 60, 1276–1279.
Wolf, B.J., Choi, J.E., and Exley, M.A. (2018). Novel approaches to exploiting invariant NKT Cells in cancer immunotherapy. Front Immunol 9, 384.
Zhang, Y., Kong, W., and Jiang, J. (2017). Prevention and treatment of cancer targeting chronic inflammation: research progress, potential agents, clinical studies and mechanisms. Sci China Life Sci 60, 601–616.
Yu, X., Xu, L., Chang, Y., Huang, X., and Zhao, X. (2018). Rapid reconstitution of NK1 cells after allogeneic transplantation is associated with a reduced incidence of graft-versus-host disease. Sci China Life Sci 61, 902–911.
Zhang, E., Gu, J., and Xu, H. (2018). Prospects for chimeric antigen receptor-modified T cell therapy for solid tumors. Mol Cancer 17, 7.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81873871) and the Fundamental Research Funds for the Central Universities (BMU2018XY001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, C., Zhang, J. Chimeric antigen receptor engineered innate immune cells in cancer immunotherapy. Sci. China Life Sci. 62, 633–639 (2019). https://doi.org/10.1007/s11427-018-9451-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-018-9451-0