Abstract
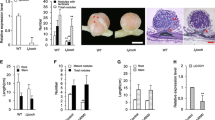
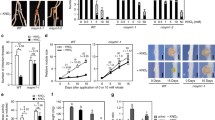
Symbiosis receptor-like kinase (SymRK) is a key protein mediating the legume-Rhizobium symbiosis. Our previous work has identified an MAP kinase kinase, SIP2, as a SymRK-interacting protein to positively regulate nodule organogenesis in Lotus japonicus, suggesting that an MAPK cascade might be involved in Rhizobium-legume symbiosis. In this study, LjMPK6 was identified as a phosphorylation target of SIP2. Stable transgenic L. japonicus with RNAi silencing of LjMPK6 decreased the numbers of nodule primordia (NP) and nodule, while plants overexpressing LjMPK6 increased the numbers of nodule, infection threads (ITs), and NP, indicating that LjMPK6 plays a positive role in nodulation. LjMPK6 could interact with a cytokinin receptor, LHK1 both in vivo and in vitro. LjMPK6 was shown to compete with LHP1 to bind to the receiver domain (RD) of LHK1and to downregulate the expression of two LjACS (1-aminocyclopropane-1-carboxylic acid synthase) genes and ethylene levels during nodulation. This study demonstrated an important role of LjMPK6 in regulation of nodule organogenesis and ethylene production in L. japonicus.
Similar content being viewed by others
References
Berriri, S., Garcia, A.V., Frei dit Frey, N., Rozhon, W., Pateyron, S., Leonhardt, N., Montillet, J.L., Leung, J., Hirt, H., and Colcombet, J. (2012). Constitutively active mitogen-activated protein kinase versions reveal functions of Arabidopsis MPK4 in pathogen defense signaling. Plant Cell 24, 4281–4293.
Brodersen, P., Petersen, M., Bjørn Nielsen, H., Zhu, S., Newman, M.A., Shokat, K.M., Rietz, S., Parker, J., and Mundy, J. (2006). Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J 47, 532–546.
Cao, Y., Halane, M.K., Gassmann, W., and Stacey, G. (2017). The role of plant innate immunity in the legume-rhizobium symbiosis. Annu Rev Plant Biol 68, 535–561.
Cardinale, F., Meskiene, I., Ouaked, F., and Hirt, H. (2002). Convergence and divergence of stress-induced mitogen-activated protein kinase signaling pathways at the level of two distinct mitogen-activated protein kinase kinases. Plant Cell 14, 703–711.
Chae, H.S., and Kieber, J.J. (2005). Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci 10, 291–296.
Chai, J., Liu, J., Zhou, J., and Xing, D. (2014). Mitogen-activated protein kinase 6 regulates NPR1 gene expression and activation during leaf senescence induced by salicylic acid. J Exp Bot 65, 6513–6528.
Chen, T., Zhou, B., Duan, L., Zhu, H., and Zhang, Z. (2017). MtMAPKK4 is an essential gene for growth and reproduction of Medicago truncatula. Physiol Plantarum 159, 492–503.
Chen, T., Zhu, H., Ke, D., Cai, K., Wang, C., Gou, H., Hong, Z., and Zhang, Z. (2012). A MAP kinase kinase interacts with SymRK and regulates nodule organogenesis in Lotus japonicus. Plant Cell 24, 823–838.
de Zelicourt, A., Colcombet, J., and Hirt, H. (2016). The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci 21, 677–685.
Dortay, H., Mehnert, N., Bürkle, L., Schmülling, T., and Heyl, A. (2006). Analysis of protein interactions within the cytokinin-signaling pathway of Arabidopsis thaliana. FEBS J 273, 4631–4644.
Endre, G., Kereszt, A., Kevei, Z., Mihacea, S., Kaló, P., and Kiss, G.B. (2002). A receptor kinase gene regulating symbiotic nodule development. Nature 417, 962–966.
Frankowski, K., Kesy, J., and Kopcewicz, J. (2007). Regulation of ethylene biosynthesis in plants. Postepy Biochem 53, 66–73.
Fukai, E., Soyano, T., Umehara, Y., Nakayama, S., Hirakawa, H., Tabata, S., Sato, S., and Hayashi, M. (2012). Establishment of a Lotus japonicus gene tagging population using the exon-targeting endogenous retrotransposon LORE1. Plant J 69, 720–730.
Gamas, P., Brault, M., Jardinaud, M.F., and Frugier, F. (2017). Cytokinins in symbiotic nodulation: when, where, what for? Trends Plant Sci 22, 792–802.
Gao, M., Liu, J., Bi, D., Zhang, Z., Cheng, F., Chen, S., and Zhang, Y. (2008). MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res 18, 1190–1198.
Gonzalez-Rizzo, S., Crespi, M., and Frugier, F. (2006). The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18, 2680–2693.
Gresshoff, P.M. (2003). Post-genomic insights into plant nodulation symbioses. Genome Biol 4, 201.
Gudesblat, G.E., Iusem, N.D., and Morris, P.C. (2007). Arabidopsis MPK3, a key signalling intermediate in stomatal function. Plant Signal Behav 2, 271–272.
Han, L., Li, G.J., Yang, K.Y., Mao, G., Wang, R., Liu, Y., and Zhang, S. (2010). Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J 48, no.
Hansen, M., Chae, H.S., and Kieber, J.J. (2009). Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J 57, 606–614.
Heidstra, R., Geurts, R., Franssen, H., Spaink, H.P., van Kammen, A., and Bisseling, T. (1994). Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol 105, 787–797.
Held, M., Hou, H., Miri, M., Huynh, C., Ross, L., Hossain, M.S., Sato, S., Tabata, S., Perry, J., Wang, T.L., et al. (2014). Lotus japonicus cytokinin receptors work partially redundantly to mediate nodule formation. Plant Cell 26, 678–694.
Hettenhausen, C., Schuman, M.C., and Wu, J. (2015). MAPK signaling: A key element in plant defense response to insects. Insect Sci 22, 157–164.
Hutchison, C.E., and Kieber, J.J. (2002). Cytokinin signaling in Arabidopsis. Plant Cell 14, S47–S59.
Hwang, I., and Sheen, J. (2001). Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413, 383–389.
Jia, W., Li, B., Li, S., Liang, Y., Wu, X., Ma, M., Wang, J., Gao, J., Cai, Y., Zhang, Y., et al. (2016). Mitogen-activated protein kinase cascade MKK7-MPK6 plays important roles in plant development and regulates shoot branching by phosphorylating PIN1 in Arabidopsis. PLoS Biol 14, e1002550.
Joo, S., Liu, Y., Lueth, A., and Zhang, S. (2008). MAPK phosphorylationinduced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J 54, 129–140.
Kiegerl, S., Cardinale, F., Siligan, C., Gross, A., Baudouin, E., Liwosz, A., Eklöf, S., Till, S., Bögre, L., Hirt, H., et al. (2000). SIMKK, a mitogenactivated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 12, 2247–2258.
Kim, C.Y., Liu, Y., Thorne, E.T., Yang, H., Fukushige, H., Gassmann, W., Hildebrand, D., Sharp, R.E., and Zhang, S. (2003). Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15, 2707–2718.
Li, G., Meng, X., Wang, R., Mao, G., Han, L., Liu, Y., and Zhang, S. (2012). Dual-level regulation of ACC synthase activity by MPK3/ MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet 8, e1002767.
Liu, J.Z., Braun, E., Qiu, W.L., Shi, Y.F., Marcelino-Guimarães, F.C., Navarre, D., Hill, J.H., and Whitham, S.A. (2014). Positive and negative roles for soybean MPK6 in regulating defense responses. MPMI 27, 824–834.
Liu, Y., and Zhang, S. (2004). Phosphorylation of 1-aminocyclopropane-1- carboxylic acid synthase by MPK6, a stress-responsive mitogenactivated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16, 3386–3399.
Lopez-Gomez, M., Sandal, N., Stougaard, J., and Boller, T. (2012). Interplay of flg22-induced defence responses and nodulation in Lotus japonicus. J Exp Bot 63, 393–401.
Lu, C., Han, M.H., Guevara-Garcia, A., and Fedoroff, N.V. (2002). Nonlinear partial differential equations and applications: Mitogenactivated protein kinase signaling in postgermination arrest of development by abscisic acid. Proc Natl Acad Sci USA 99, 15812–15817.
Madsen, E.B., Madsen, L.H., Radutoiu, S., Olbryt, M., Rakwalska, M., Szczyglowski, K., Sato, S., Kaneko, T., Tabata, S., Sandal, N., et al. (2003). A receptor kinase gene of the LysM type is involved in legumeperception of rhizobial signals. Nature 425, 637–640.
Maekawa, T., Kusakabe, M., Shimoda, Y., Sato, S., Tabata, S., Murooka, Y., and Hayashi, M. (2008). Polyubiquitin promoter-based binary vectors for overexpression and gene silencing in Lotus japonicus. MPMI 21, 375–382.
Mähönen, A.P., Higuchi, M., Törmäkangas, K., Miyawaki, K., Pischke, M. S., Sussman, M.R., Helariutta, Y., and Kakimoto, T. (2006). Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr Biol 16, 1116–1122.
Mao, G., Meng, X., Liu, Y., Zheng, Z., Chen, Z., and Zhang, S. (2011). Phosphorylation of a WRKY transcription factor by two pathogenresponsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23, 1639–1653.
Meldau, S., Ullman-Zeunert, L., Govind, G., Bartram, S., and Baldwin, I.T. (2012). MAPK-dependent JA and SA signalling in Nicotiana attenuata affects plant growth and fitness during competition with conspecifics. BMC Plant Biol 12, 213.
Meng, X., Xu, J., He, Y., Yang, K.Y., Mordorski, B., Liu, Y., and Zhang, S. (2013). Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 25, 1126–1142.
Meng, Y., Ma, N., Zhang, Q., You, Q., Li, N., Ali Khan, M., Liu, X., Wu, L., Su, Z., and Gao, J. (2014). Precise spatio-temporal modulation of ACC synthase by MPK6 cascade mediates the response of rose flowers to rehydration. Plant J 79, 941–950.
Miller, J.B., Pratap, A., Miyahara, A., Zhou, L., Bornemann, S., Morris, R. J., and Oldroyd, G.E.D. (2013). Calcium/Calmodulin-dependent protein kinase is negatively and positively regulated by calcium, providing a mechanism for decoding calcium responses during symbiosis signaling. Plant Cell 25, 5053–5066.
Minkenberg, B., Xie, K., and Yang, Y. (2017). Discovery of rice essential genes by characterizing a CRISPR-edited mutation of closely related rice MAP kinase genes. Plant J 89, 636–648.
Miri, M., Janakirama, P., Held, M., Ross, L., and Szczyglowski, K. (2016). Into the root: how cytokinin controls rhizobial infection. Trends Plant Sci 21, 178–186.
Miyata, K., Kawaguchi, M., and Nakagawa, T. (2013). Two distinct EIN2 genes cooperatively regulate ethylene signaling in Lotus japonicus.. Plant Cell Physiol 54, 1469–1477.
Morieri, G., Martinez, E.A., Jarynowski, A., Driguez, H., Morris, R., Oldroyd, G.E.D., and Downie, J.A. (2013). Host-specific Nod-factors associated with Medicago truncatula nodule infection differentially induce calcium influx and calcium spiking in root hairs. New Phytol 200, 656–662.
Mortier, V., Holsters, M., and Goormachtig, S. (2012). Never too many? How legumes control nodule numbers. Plant Cell Environ 35, 245–258.
Moussatche, P., and Klee, H.J. (2004). Autophosphorylation activity of the Arabidopsis ethylene receptor multigene family. J Biol Chem 279, 48734–48741.
Murray, J.D., Karas, B.J., Sato, S., Tabata, S., Amyot, L., and Szczyglowski, K. (2007). A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315, 101–104.
Nakagami, H., Pitzschke, A., and Hirt, H. (2005). Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 10, 339–346.
Neupane, A., Nepal, M.P., Benson, B.V., Macarthur, K.J., and Piya, S. (2013). Evolutionary history of mitogen-activated protein kinase (MAPK) genes in Lotus, Medicago, and Phaseolus. Plant Signal Behav 8, e27189.
Nie, S., and Xu, H. (2016). Riboflavin-induced disease resistance requires the mitogen-activated protein kinases 3 and 6 in Arabidopsis thaliana. PLoS ONE 11, e0153175.
Oldroyd, G.E.D., Engstrom, E.M., and Long, S.R. (2001). Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell 13, 1835–1849.
Penmetsa, R.V., and Cook, D.R. (1997). A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275, 527–530.
Pitzschke, A. (2015). Modes of MAPK substrate recognition and control. Trends Plant Sci 20, 49–55.
Punwani, J.A., Hutchison, C.E., Schaller, G.E., and Kieber, J.J. (2010). The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling. Plant J 62, 473–482.
Radutoiu, S., Madsen, L.H., Madsen, E.B., Felle, H.H., Umehara, Y., Grønlund, M., Sato, S., Nakamura, Y., Tabata, S., Sandal, N., et al. (2003). Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425, 585–592.
Rasmussen, M.W., Roux, M., Petersen, M., and Mundy, J. (2012). MAP kinase cascades in Arabidopsis innate immunity. Front Plant Sci 3, 169.
Reid, D., Liu, H., Kelly, S., Kawaharada, Y., Mun, T., Andersen, S.U., Desbrosses, G., and Stougaard, J. (2018). Dynamics of ethylene production in response to compatible Nod factor. Plant Physiol 176, 1764–1772.
Ryu, H., Laffont, C., Frugier, F., and Hwang, I. (2017). MAP kinasemediated negative regulation of symbiotic nodule formation in Medicago truncatula. Mol Cells 40, 17–23.
Sasaki, T., Suzaki, T., Soyano, T., Kojima, M., Sakakibara, H., and Kawaguchi, M. (2014). Shoot-derived cytokinins systemically regulate root nodulation. Nat Commun 5, 4983.
Schauser, L., Handberg, K., Sandal, N., Stiller, J., Thykjaer, T., Pajuelo, E., Nielsen, A., and Stougaard, J. (1998). Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol Gen Genet 259, 414–423.
Singh, P., Mohanta, T.K., and Sinha, A.K. (2015). Unraveling the intricate nexus of molecular mechanisms governing rice root development: OsMPK3/6 and auxin-cytokinin interplay. PLoS ONE 10, e0123620.
Stanko, V., Giuliani, C., Retzer, K., Djamei, A., Wahl, V., Wurzinger, B., Wilson, C., Heberle-Bors, E., Teige, M., and Kragler, F. (2015). Timing is everything: highly specific and transient expression of a MAP kinase determines auxin-induced leaf venation patterns in Arabidopsis. Mol Plant 8, 829.
Takahashi, F., Yoshida, R., Ichimura, K., Mizoguchi, T., Seo, S., Yonezawa, M., Maruyama, K., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2007). The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19, 805–818.
Tanaka, Y., Suzuki, T., Yamashino, T., and Mizuno, T. (2004). Comparative studies of the AHP histidine-containing phosphotransmitters implicated in His-to-Asp phosphorelay in Arabidopsis thaliana. Biosci Biotech Biochem 68, 462–465.
Tirichine, L., Sandal, N., Madsen, L.H., Radutoiu, S., Albrektsen, A.S., Sato, S., Asamizu, E., Tabata, S., and Stougaard, J. (2007). A gain-offunction mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315, 104–107.
Urbanski, D.F., Malolepszy, A., Stougaard, J., and Andersen, S.U. (2012). Genome-wide LORE1 retrotransposon mutagenesis and highthroughput insertion detection in Lotus japonicus. Plant J 69, 731–741.
van Zeijl, A., Op den Camp, R.H.M., Deinum, E.E., Charnikhova, T., Franssen, H., Op den Camp, H.J.M., Bouwmeester, H., Kohlen, W., Bisseling, T., and Geurts, R. (2015). Rhizobium lipochitooligosaccharide signaling triggers accumulation of cytokinins in Medicago truncatula roots. Mol Plant 8, 1213–1226.
Verma, V., Sivaraman, J., Srivastava, A.K., Sadanandom, A., and Kumar, P. P. (2015). Destabilization of interaction between cytokinin signaling intermediates AHP1 and ARR4 modulates Arabidopsis development. New Phytol 206, 726–737.
Vijn, I., Das Neves, L., van Kammen, A., Franssen, H., and Bisseling, T. (1993). Nod factors and nodulation in plants. Science 260, 1764–1765.
Wang, C., Yu, H., Luo, L., Duan, L., Cai, L., He, X., Wen, J., Mysore, K.S., Li, G., Xiao, A., et al. (2016). NODULES WITH ACTIVATED DEFENSE 1 is required for maintenance of rhizobial endosymbiosis in Medicago truncatula. New Phytol 212, 176–191.
Wang, C., Zhu, M., Duan, L., Yu, H., Chang, X., Li, L., Kang, H., Feng, Y., Zhu, H., Hong, Z., et al. (2015). Lotus japonicus clathrin heavy Chain1 is associated with Rho-like GTPase ROP6 and involved in nodule formation. Plant Physiol 167, 1497–1510.
Wang, H., Ngwenyama, N., Liu, Y., Walker, J.C., and Zhang, S. (2007). Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19, 63–73.
Wang, P., and Zhu, J.K. (2016). Assessing kinase activity in plants with ingel kinase assays. Methods Mol Biol 1363, 189–197.
Wopereis, J., Pajuelo, E., Dazzo, F.B., Jiang, Q., Gresshoff, P.M., de Bruijn, F.J., Stougaard, J., and Szczyglowski, K. (2000). Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23, 97–114.
Xu, J., Meng, J., Meng, X., Zhao, Y., Liu, J., Sun, T., Liu, Y., Wang, Q., and Zhang, S. (2016). Pathogen-responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity. Plant Cell 28, 1144–1162.
Xu, J., and Zhang, S. (2015). Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci 20, 56–64.
Yi, J., Lee, Y.S., Lee, D.Y., Cho, M.H., Jeon, J.S., and An, G. (2016). OsMPK6 plays a critical role in cell differentiation during early embryogenesis in Oryza sativa. J Exp Bot 67, 2425–2437.
Zhou, C., Cai, Z., Guo, Y., and Gan, S. (2009). An Arabidopsis mitogenactivated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol 150, 167–177.
Zhu, H., Chen, T., Zhu, M., Fang, Q., Kang, H., Hong, Z., and Zhang, Z. (2008). A novel ARID DNA-binding protein interacts with SymRK and is expressed during early nodule development in Lotus japonicus. Plant Physiol 148, 337–347.
Zong, W., Tang, N., Yang, J., Peng, L., Ma, S., Xu, Y., Li, G., and Xiong, L. (2016). Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought resistance related genes. Plant Physiol 171, 2810–2825.
Acknowledgements
We thank Dr. K. Szczyglowski (Agriculture and Agri- Food Canada, University of Western Ontario, Canada) for kindly providing LHK1 mutants, Dr. A. Downie (John Innes Centre) for providing M. loti strain R7A carrying pMP2112, Dr. G. Wu (Shanghai Jiao Tong University, China) for providing M. loti MAFF303099, Dr. S. Wang (Huazhong Agricultural University, China) for providing pCAMBIA1301U, L. japonicus LORE1 mutant collection (Centre for Carbohydrate Recongnition and Signaling, Aarhus University, Denmark) for providing LjMPK6 mutants. This work was supported by the National Key R&D Program of China (2016YF0100700), the National Natural Science Foundation of China (31670240 and 31870219), the State Key Laboratory of Agricultural Microbiology (AMLKF201503 and AMLKF201608), the Graduate Education Innovation Fund of Huazhong Agricultural University (to Z.Z.), and Graduate Student Research Innovation Project of Huazhong Agricultural University (to J.Y.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Electronic supplementary material

Figure S1
Expression levels of NIN in L. japonicus.

Figure S2
GUS activity analysis in stable transgenic L. japonicus plants expressing LjMPK6pro:GUS.

Figure S3
Characterization of an LjMPK6 LORE1 mutant.

Figure S4
Gus staining in transgenic plants expressing GUS reporter under the control of LjNAD1 promoter or maize ubiquitin promoter.


Figure S6
Expression of LjMPK6 in LjMPK6-ox stable transgenic plants under the control of L. japonicus ubiquitin promoter.

Figure S7
Immunoblot analysis of protein expression in yeast cells.

Figure S8
Competition of BSA with LHP1 for binding to LHK1.

Figure S9
Expression of NIN and NSP2 in LjMPK6-ox and LjMPK6-RNAi transgenic plants during nodulation.
Table S1
Primers used in this study
Table S2
Accession numbers of genes used in this study
Rights and permissions
About this article
Cite this article
Yin, J., Guan, X., Zhang, H. et al. An MAP kinase interacts with LHK1 and regulates nodule organogenesis in Lotus japonicus. Sci. China Life Sci. 62, 1203–1217 (2019). https://doi.org/10.1007/s11427-018-9444-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-018-9444-9