Abstract
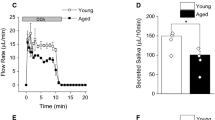
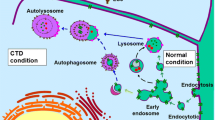
Autophagy is a catabolic process which is involved in the development of many diseases including diabetes mellitus and its complications. Hyposalivation is a common complication of diabetes mellitus, whereas its mechanism remains unclear. Here, we observed that the stimulated salivary flow rate of SMG was significantly decreased in db/db mice, a diabetic mice model. The expressions of aquaporin 5 (AQP5), a water channel protein, were decreased, whereas the mRNA level of AQP5 was increased in SMGs of both diabetic patients and mice. Under transmission electron microcope, more autophagosomes were detected in diabetic SMGs. Expressions of autophagy related proteins LC3II, Beclin-1 and ATG5 were increased, meanwhile autophagy substrate p62 was decreased in SMGs of diabetic patients and mice, indicating that autophagy was activated in diabetic SMG. Double immunofluorescence staining showed that the colocalization of AQP5 and LC3 was increased in SMGs of diabetic mice. In cultured SMG-C6 cells, high glucose (HG), but not high osmotic pressure, reduced AQP5 protein expression and induced autophagy. Moreover, inhibition of autophagy by 3-methyladenin, an autophagy inhibitor, or by autophagy-related gene 5 siRNA, decreased HG-induced AQP5 reduction in SMG-C6 cells. Additionally, the expression of p-p85, p-Akt and p-mTOR were decreased in HG-treated SMG-C6 cells. Pretreatment with 740Y-P, a PI3K agonist, significantly suppressed HG-induced autophagy and AQP5 degradation. Taken together, these results indicate that autophagy plays a crucial role in AQP5 degradation in diabetic SMG via PI3K/Akt/mTOR signaling pathway, which contributes to the dysfunction of diabetic SMG. Our study provides a novel mechanism of diabetic hyposalivation.
Similar content being viewed by others
References
Abada, A., and Elazar, Z. (2014). Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep 15, 839–852.
Abd-Elraheem, S.E., El Saeed, A.M., and Mansour, H.H. (2017). Salivary changes in type 2 diabetic patients. Diabetes Metab Syndrome Clin Res Rev 11, S637–S641.
Alam, J., Koh, J.H., Kwok, S.K., Park, S.H., Park, K., and Choi, Y. (2017). Functional epitopes for anti-aquaporin 5 antibodies in Sjögren syndrome. J Dent Res 96, 1414–1421.
Azlina, A., Javkhlan, P., Hiroshima, Y., Hasegawa, T., Yao, C., Akamatsu, T., and Hosoi, K. (2010). Roles of lysosomal proteolytic systems in AQP5 degradation in the submandibular gland of rats following chorda tympani parasympathetic denervation. Am J Physiol Gastrointestinal Liver Physiol 299, G1106–G1117.
Babu, N., Masthan, K., Bhattacharjee, T., and Elumalai, M. (2014). Salivathe key regulator of oral changes in diabetes patients. Int J Pharmaceut Sci Res 5, 2579–2583.
Chuang, S.F., Sung, J.M., Kuo, S.C., Huang, J.J., and Lee, S.Y. (2005). Oral and dental manifestations in diabetic and nondiabetic uremic patients receiving hemodialysis. Oral Surgery Oral Med Oral Pathol Oral Rad Endodontol 99, 689–695.
Cong, X., Zhang, Y., Li, J., Mei, M., Ding, C., Xiang, R.L., Zhang, L.W., Wang, Y., Wu, L.L., and Yu, G.Y. (2015). Claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptor in epithelial cells. J Cell Sci 128, 2271–2286.
Garrett, J.R. (1987). The proper role of nerves in salivary secretion: a review. J Dent Res 66, 387–397.
Hosoi, K. (2016). Physiological role of aquaporin 5 in salivary glands. Pflugers Arch 468, 519–539.
Huang, C., Lin, M.Z., Cheng, D., Braet, F., Pollock, C.A., and Chen, X.M. (2016). KCa3.1 mediates dysfunction of tubular autophagy in diabetic kidneys via PI3k/Akt/mTOR signaling pathways. Sci Rep 6, 23884.
Jung, H.S., and Lee, M.S. (2010). Role of autophagy in diabetes and mitochondria. Ann New York Acad Sci 1201, 79–83.
Karabasil, M.R., Hasegawa, T., Azlina, A., Purwanti, N., Yao, C., Akamatsu, T., Tomioka, S., and Hosoi, K. (2011). Effects of naturally occurring G103D point mutation of AQP5 on its water permeability, trafficking and cellular localization in the submandibular gland of rats. Biol Cell 103, 69–86.
Kilkenny, C., Browne, W., Cuthill, I.C., Emerson, M., Altman, D.G., and Altman, D.G. (2010). Animal research: reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol 160, 1577–1579.
Kim, J., Cheon, H., Jeong, Y.T., Quan, W., Kim, K.H., Cho, J.M., Lim, Y. M., Oh, S.H., Jin, S.M., Kim, J.H., et al. (2014). Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes. J Clin Invest 124, 3311–3324.
Kong, C.G., Park, J.B., Kim, M.S., and Park, E.Y. (2014). High glucose accelerates autophagy in adult rat intervertebral disc cells. Asian Spine J 8, 543–548.
Krane, C.M., Melvin, J.E., Nguyen, H.V., Richardson, L., Towne, J.E., Doetschman, T., and Menon, A.G. (2001). Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem 276, 23413–23420.
Li, S., and Le, W. (2017). An insight review of autophagy biology and neurodegenerative diseases: machinery, mechanisms and regulation. Sci China Life Sci 60, 1457–1459.
Li, Z., Zhao, D., Gong, B., Xu, Y., Sun, H., Yang, B., and Zhao, X. (2006). Decreased saliva secretion and down-regulation of AQP5 in submandibular gland in irradiated rats. Radiat Res 165, 678–687.
Lilliu, M.A., Solinas, P., Cossu, M., Puxeddu, R., Loy, F., Isola, R., Quartu, M., Melis, T., and Isola, M. (2015). Diabetes causes morphological changes in human submandibular gland: a morphometric study. J Oral Pathol Med 44, 291–295.
Lima, D.L.F., Carneiro, S.D.R.M., Barbosa, F.T.S., Saintrain, M.V.L., Moizan, J.A.H., and Doucet, J. (2017). Salivary flow and xerostomia in older patients with type 2 diabetes mellitus. PLoS ONE 12, e0180891.
Liu, J., Tang, Y., Feng, Z., Hou, C., Wang, H., Yan, J., Liu, J., Shen, W., Zang, W., Liu, J., et al. (2014). Acetylated FoxO1 mediates high-glucose induced autophagy in H9c2 cardiomyoblasts: regulation by a polyphenol-(−)-epigallocatechin-3-gallate. Metabolism 63, 1314–1323.
Liu, N., Shi, Y., and Zhuang, S. (2016). Autophagy in chronic kidney diseases. Kidney Dis 2, 37–45.
Ma, T., Song, Y., Gillespie, A., Carlson, E.J., Epstein, C.J., and Verkman, A.S. (1999). Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem 274, 20071–20074.
Ma, T., Zhu, J., Chen, X., Zha, D., Singhal, P.C., and Ding, G. (2013). High glucose induces autophagy in podocytes. Exp Cell Res 319, 779–789.
Mandel, I.D. (1989). The role of saliva in maintaining oral homeostasis. J Am Dent Assoc 119, 298–304.
Mathiassen, S.G., De Zio, D., and Cecconi, F. (2017). Autophagy and the cell cycle: a complex landscape. Front Oncol 7, 51.
Morgan-Bathke, M., Lin, H.H., Ann, D.K., and Limesand, K.H. (2015). The role of autophagy in salivary gland homeostasis and stress responses. J Dent Res 94, 1035–1040.
Munasinghe, P.E., Riu, F., Dixit, P., Edamatsu, M., Saxena, P., Hamer, N.S. J., Galvin, I.F., Bunton, R.W., Lequeux, S., Jones, G., et al. (2016). Type-2 diabetes increases autophagy in the human heart through promotion of Beclin-1 mediated pathway. Int J Cardiol 202, 13–20.
Murdiastuti, K., Purwanti, N., Karabasil, M.R., Li, X., Yao, C., Akamatsu, T., Kanamori, N., and Hosoi, K. (2006). A naturally occurring point mutation in the rat aquaporin 5 gene, influencing its protein production by and secretion of water from salivary glands. Am J Physiol Gastrointest Liver Physiol 291, G1081–G1088.
Proctor, G.B., and Carpenter, G.H. (2014). Salivary secretion: mechanism and neural regulation. Monogr Oral Sci 24, 14–29.
Riahi, Y., Wikstrom, J.D., Bachar-Wikstrom, E., Polin, N., Zucker, H., Lee, M.S., Quan, W., Haataja, L., Liu, M., Arvan, P., et al. (2016). Autophagy is a major regulator of beta cell insulin homeostasis. Diabetologia 59, 1480–1491.
Robinson, M.M., Dasari, S., Karakelides, H., Bergen 3rd, H.R., and Nair, K.S. (2016). Release of skeletal muscle peptide fragments identifies individual proteins degraded during insulin deprivation in type 1 diabetic humans and mice. Am J Physiol Endocrinol Metab 311, e628–E637.
Sinha, S., and Levine, B. (2008). The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene 27 Suppl 1, S137–S148.
Sridhar, S., Botbol, Y., Macian, F., and Cuervo, A.M. (2012). Autophagy and disease: always two sides to a problem. J Pathol 226, 255–273.
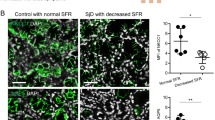
Steinfeld, S., Cogan, E., King, L.S., Agre, P., Kiss, R., and Delporte, C. (2001). Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjögren’s syndrome patients. Lab Invest 81, 143–148.
Wang, X., Feng, Z., Li, J., Chen, L., and Tang, W. (2016). High glucose induces autophagy of MC3T3-E1 cells via ROS-AKT-mTOR axis. Mol Cell Endocrinol 429, 62–72.
Xiao, E., Mattos, M., Vieira, G.H.A., Chen, S., Corrêa, J.D., Wu, Y., Albiero, M.L., Bittinger, K., and Graves, D.T. (2017). Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe 22, 120–128.e4.
Xu, H., Shan, X.F., Cong, X., Yang, N.Y., Wu, L.L., Yu, G.Y., Zhang, Y., and Cai, Z.G. (2015). Pre-and post-synaptic effects of botulinum toxin a on submandibular glands. J Dent Res 94, 1454–1462.
Yang, J.S., Lu, C.C., Kuo, S.C., Hsu, Y.M., Tsai, S.C., Chen, S.Y., Chen, Y. T., Lin, Y.J., Huang, Y.C., Chen, C.J., et al. (2017). Autophagy and its link to type II diabetes mellitus. Biomedicine (Taipei) 7, 1–12.
Yao, F., Zhang, M., and Chen, L. (2016). 5′-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta Pharmaceut Sin B 6, 20–25.
Yin, Z., Pascual, C., and Klionsky, D.J. (2016). Autophagy: machinery and regulation. Microb Cell 3, 588–596.
Acknowledgements
We thank Dr. David O. Quissell (University of Colorado Health Science Center, USA) for the generous gift of rat SMG-C6 cell line. We would like to thank Jiazeng Su, Wenxuan Zhu, Kefu Zhang, Yanyan Zhang for their work in the collection of patients’ information. This work was supported by National Natural Science Foundation of China (81570993, 81671005) and Beijing Natural Science Foundation of China (7162100).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, Y., Shi, X., Mao, Q. et al. Aquaporin 5 is degraded by autophagy in diabetic submandibular gland. Sci. China Life Sci. 61, 1049–1059 (2018). https://doi.org/10.1007/s11427-018-9318-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-018-9318-8