Abstract
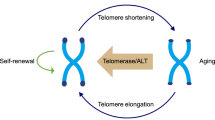
Pluripotent or multipotent stem cells are involved in development and tissue homeostasis; they have the ability to self-renew and differentiate into various types of functional cells. To maintain these properties, stem cells must undergo sustained or unlimited proliferation that requires the stabilization of telomeres, which are essential for chromosome end protection. Telomerase, an RNA-dependent DNA polymerase, synthesizes telomeric DNA. Through the lengthening of telomeres the lifespans of cells are extended, or indefinite proliferation is conferred; this is intimately associated with stem cell phenotype. This review highlights our current understanding of telomerase as a “stemness” enzyme and discusses the underlying implications.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Blackburn EH. Switching and signaling at the telomere. Cell, 2001, 106: 661–673
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell, 2013, 153: 1194–1217
Cheng G, Kong F, Luan Y, Sun C, Wang J, Zhang L, Jiang B, Qi T, Zhao J, Zheng C, Xu D. Differential shortening rate of telomere length in the development of human fetus. Biochem Biophys Res Commun, 2013, 442: 112–115
Holmes DK, Bellantuono I, Walkinshaw SA, Alfirevic Z, Johnston TA, Subhedar NV, Chittick R, Swindell R, Wynn RF. Telomere length dynamics differ in foetal and early post-natal human leukocytes in a longitudinal study. Biogerontology, 2009, 10: 279–284
Shay JW, Wright WE. Hallmarks of telomeres in ageing research. J Pathol, 2007, 211: 114–123
Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT). Gene, 2012, 498: 135–146
Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev, 2002, 66: 407–425
Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell, 2008, 132: 681–696
Rando TA. Stem cells, ageing and the quest for immortality. Nature, 2006, 441: 1080–1086
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007, 131: 861–872
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006, 126: 663–676
Buganim Y, Faddah DA, Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat Rev Genet, 2013, 14: 427–439
Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med, 2009, 15: 1010–1012
Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell, 2012, 10: 717–728
Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science, 2007, 315: 1850–1853
Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature, 1999, 402: 551–555
Vulliamy T, Dokal I. Dyskeratosis congenita. Semin Hematol, 2006, 43: 157–166
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science, 1998, 279: 349–352
Hou M, Wang X, Popov N, Zhang A, Zhao X, Zhou R, Zetterberg A, Björkholm M, Henriksson M, Gruber A, Xu D. The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp Cell Res, 2002, 274: 25–34
Shay JW, Wright WE. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett, 2010, 584: 3819–3825
Zeng X. Human embryonic stem cells: mechanisms to escape replicative senescence? Stem Cell Rev, 2007, 3: 270–279
Yang C, Przyborski S, Cooke MJ, Zhang X, Stewart R, Anyfantis G, Atkinson SP, Saretzki G, Armstrong L, Lako M. A key role for telomerase reverse transcriptase unit in modulating human embryonic stem cell proliferation, cell cycle dynamics, and in vitro differentiation. Stem Cells, 2008, 26: 850–863
Armstrong L, Lako M, Lincoln J, Cairns PM, Hole N. mTert expression correlates with telomerase activity during the differentiation of murine embryonic stem cells. Mech Dev, 2000, 97: 109–116
Wong CW, Hou PS, Tseng SF, Chien CL, Wu KJ, Chen HF, Ho HN, Kyo S, Teng SC. Kruppel-like transcription factor 4 contributes to maintenance of telomerase activity in stem cells. Stem Cells, 2010, 28: 1510–1517
Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science, 2013, 336: 1549–1554
Zhang Y, Toh L, Lau P, Wang X. Telomerase reverse transcriptase (TERT) is a novel target of Wnt/beta-catenin pathway in human cancer. J Biol Chem, 2012, 287: 32494–324511
Coussens M, Davy P, Brown L, Foster C, Andrews WH, Nagata M, Allsopp R. RNAi screen for telomerase reverse transcriptase transcriptional regulators identifies HIF1alpha as critical for telomerase function in murine embryonic stem cells. Proc Natl Acad Sci USA, 2010, 107: 13842–13847
Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol, 2007, 8: 703–713
Flores I, Canela A, Vera E, Tejera A, Cotsarelis G, Blasco MA. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev, 2008, 22: 654–667
Chiu CP, Dragowska W, Kim NW, Vaziri H, Yui J, Thomas TE, Harley CB, Lansdorp PM. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells, 1996, 14: 239–248
Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA, 1994, 91: 9857–9860
Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity, 1996, 5: 207–216
Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature, 2001, 413: 432–435
Marrone A, Walne A, Tamary H, Masunari Y, Kirwan M, Beswick R, Vulliamy T, Dokal I. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood, 2007, 110: 4198–4205
Calado RT, Young NS. Telomere diseases. N Engl J Med, 2009, 361: 2353–2365
Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet, 2002, 359: 2168–2170
Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med, 2005, 352: 1413–1424
Pignolo RJ, Suda RK, McMillan EA, Shen J, Lee SH, Choi Y, Wright AC, Johnson FB. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell, 2008, 7: 23–31
Pucci F, Gardano L, Harrington L. Short telomeres in ESCs lead to unstable differentiation. Cell Stem Cell, 2013, 12: 479–486
Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J, 1999, 18: 2950–2960
Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med, 2007, 13: 742–747
Cong Y, Shay JW. Actions of human telomerase beyond telomeres. Cell Res, 2008, 18: 725–732
Armstrong L, Saretzki G, Peters H, Wappler I, Evans J, Hole N, von Zglinicki T, Lako M. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells, 2005, 23: 516–529
Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science, 2005, 309: 1253–1256
Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, Artandi MK, Oro AE, Artandi SE. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature, 2005, 436: 1048–1052
Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, McLaughlin M, Veenstra TD, Nusse R, McCrea PD, Artandi SE. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature, 2009, 460: 66–72
Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature, 2011, 470: 359–365
Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature, 2010, 464: 520–528
Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science, 2008, 322: 1811–1815
Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature, 1996, 380: 64–66
Suhr ST, Chang EA, Rodriguez RM, Wang K, Ross PJ, Beyhan Z, Murthy S, Cibelli JB. Telomere dynamics in human cells reprogrammed to pluripotency. PLoS ONE, 2009, 4: e8124
Huang J, Wang F, Okuka M, Liu N, Ji G, Ye X, Zuo B, Li M, Liang P, Ge WW, Tsibris JC, Keefe DL, Liu L. Association of telomere length with authentic pluripotency of ES/iPS cells. Cell Res, 2013, 21: 779–792
Agarwal S, Loh YH, McLoughlin EM, Huang J, Park IH, Miller JD, Huo H, Okuka M, Dos Reis RM, Loewer S, Ng HH, Keefe DL, Goldman FD, Klingelhutz AJ, Liu L, Daley GQ. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature, 2010, 464: 292–296
Mathew R, Jia W, Sharma A, Zhao Y, Clarke LE, Cheng X, Wang H, Salli U, Vrana KE, Robertson GP, Zhu J, Wang S. Robust activation of the human but not mouse telomerase gene during the induction of pluripotency. FASEB J, 2012, 24: 2702–2715
Winkler T, Hong SG, Decker JE, Morgan MJ, Wu C, Hughes WM 5th, Yang Y, Wangsa D, Padilla-Nash HM, Ried T, Young NS, Dunbar CE, Calado RT. Defective telomere elongation and hematopoiesis from telomerase-mutant aplastic anemia iPSCs. J Clin Invest, 2013, 123: 1952–1963
Batista LF, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, Crary SM, Choi J, Sebastiano V, Cherry A, Giri N, Wernig M, Alter BP, Cech TR, Savage SA, Reijo Pera RA, Artandi SE. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature, 2011, 474: 399–402
Xin HW, Hari DM, Mullinax JE, Ambe CM, Koizumi T, Ray S, Anderson AJ, Wiegand GW, Garfield SH, Thorgeirsson SS, Avital I. Tumor-initiating label-retaining cancer cells in human gastrointestinal cancers undergo asymmetric cell division. Stem Cells, 2012, 30: 591–598
Castelo-Branco P, Zhang C, Lipman T, Fujitani M, Hansford L, Clarke I, Harley CB, Tressler R, Malkin D, Walker E, Kaplan DR, Dirks P, Tabori U. Neural tumor-initiating cells have distinct telomere maintenance and can be safely targeted for telomerase inhibition. Clin Cancer Res, 2011, 17: 111–121
Shervington A, Lu C, Patel R, Shervington L. Telomerase downregulation in cancer brain stem cell. Mol Cell Biochem, 2009, 331: 153–159
Marian CO, Wright WE, Shay JW. The effects of telomerase inhibition on prostate tumor-initiating cells. Int J Cancer, 2010, 127: 321–331
Marian CO, Cho SK, McEllin BM, Maher EA, Hatanpaa KJ, Madden CJ, Mickey BE, Wright WE, Shay JW, Bachoo RM. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumorinitiating cells leading to decreased proliferation and tumor growth. Clin Cancer Res, 2010, 16: 154–163
Muntoni A, Reddel RR. The first molecular details of ALT in human tumor cells. Hum Mol Genet, 2005, 14(Spec No. 2): R191–196
Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet, 2010, 11: 319–330
Silvestre DC, Pineda JR, Hoffschir F, Studler JM, Mouthon MA, Pflumio F, Junier MP, Chneiweiss H, Boussin FD. Alternative lengthening of telomeres in human glioma stem cells. Stem Cells, 2011, 29: 440–451
Liu Z, Li Q, Li K, Chen L, Li W, Hou M, Liu T, Yang J, Lindvall C, Björkholm M, Jia J, Xu D. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene, 2013, 32: 4203–4213
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 2008, 133: 704–715
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at link.springer.com
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kong, F., Zheng, C. & Xu, D. Telomerase as a “stemness” enzyme. Sci. China Life Sci. 57, 564–570 (2014). https://doi.org/10.1007/s11427-014-4666-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-014-4666-6