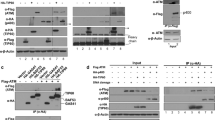
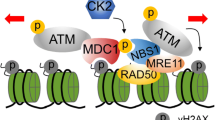
Abstract
The tumor suppressor p53 is a critical component of the DNA damage response pathway that induces a set of genes responsible for cell cycle arrest, senescence, apoptosis, and DNA repair. The ataxia telangiectasia mutated protein kinase (ATM) responds to DNA-damage stimuli and signals p53 stabilization and activation, thereby facilitating transactivation of p53 inducible genes and maintainence of genome integrity. In this study, we identified a CXXC zinc finger domain containing protein termed CF5 as a critical component in the DNA damage signaling pathway. CF5 induces p53 transcriptional activity and apoptosis in cells expressing wild type p53 but not in p53-deficient cells. Knockdown of CF5 inhibits DNA damage-induced p53 activation as well as cell cycle arrest. Furthermore, CF5 physically interacts with ATM and is required for DNA damage-induced ATM phosphorylation but not its recruitment to chromatin. These findings suggest that CF5 plays a crucial role in ATM-p53 signaling in response to DNA damage.
Similar content being viewed by others
References
Vousden K H. p53: death star. Cell, 2000, 103(5): 691–694, 11114324, 10.1016/S0092-8674(00)00171-9, 1:CAS:528:DC%2BD3cXos1Snuro%3D
Sharpless N E, DePinho R A. p53: good cop/bad cop. Cell, 2002, 110(1): 9–12, 12150992, 10.1016/S0092-8674(02)00818-8, 1:CAS:528:DC%2BD38XlsV2mtrY%3D
Lombard D B, Chua K F, Mostoslavsky R, et al. DNA repair, genome stability, and aging. Cell, 2005, 120(4): 497–512, 15734682, 10.1016/j.cell.2005.01.028, 1:CAS:528:DC%2BD2MXitVWnurY%3D
Aylon Y, Oren M. Living with p53, dying of p53. Cell, 2007, 130(4): 597–600, 17719538, 10.1016/j.cell.2007.08.005, 1:CAS:528:DC%2BD2sXhtVels77E
Marine J C, Francoz S, Maetens M, et al. Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ, 2006, 13(6): 927–934, 16543935, 10.1038/sj.cdd.4401912, 1:CAS:528:DC%2BD28Xks1Ortbo%3D
Toledo F, Wahl G M. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer, 2006, 6(12): 909–923, 17128209, 10.1038/nrc2012, 1:CAS:528:DC%2BD28Xht1Clsr3E
Vousden K H, Lane D P. p53 in health and disease. Nat Rev Mol Cell Biol, 2007, 8(4):275–283, 17380161, 10.1038/nrm2147, 1:CAS:528:DC%2BD2sXjtlygsr0%3D
Borkowska E, Binka-Kowalska A, Constantinou M, et al. P53 mutations in urinary bladder cancer patients from Central Poland. J Appl Genet, 2007, 48(2): 177–183, 17495352
Caulin C, Nguyen T, Lang G A, et al. An inducible mouse model for skin cancer reveals distinct roles for gain- and loss-of-function p53 mutations. J Clin Invest, 2007, 117(7): 1893–1901, 17607363, 10.1172/JCI31721, 1:CAS:528:DC%2BD2sXnvVansrY%3D
Gottschalg E, Scott G B, Burns P A, et al. Potassium diazoacetate-induced p53 mutations in vitro in relation to formation of O6-carboxymethyl- and O6-methyl-2′-deoxyguanosine DNA adducts: relevance for gastrointestinal cancer. Carcinogenesis, 2007, 28(2): 356–362, 16926174, 10.1093/carcin/bgl150, 1:CAS:528:DC%2BD2sXhs1Srsro%3D
Kouidou S, Malousi A, Kyventidis A, et al. G: C > A: T mutations and potential epigenetic regulation of p53 in breast cancer. Breast Cancer Res Treat, 2007, 106(3): 351–360, 17505880, 10.1007/s10549-007-9514-y, 1:CAS:528:DC%2BD2sXht1Cgt7fN
Queille S, Luron L, Spatz A, et al. Analysis of skin cancer risk factors in immunosuppressed renal transplant patients shows high levels of UV-specific tandem CC to TT mutations of the p53 gene. Carcinogenesis, 2007, 28(3): 724–731, 17065198, 10.1093/carcin/bgl191, 1:CAS:528:DC%2BD2sXjslOns7k%3D
Canman C E, Lim D S, Cimprich K A, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science, 1998, 281(5383): 1677–1679, 9733515, 10.1126/science.281.5383.1677, 1:CAS:528:DyaK1cXmtVWhtbc%3D
Lee J H, Paull T T. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science, 2004, 304(5667): 93–96, 15064416, 10.1126/science.1091496, 1:CAS:528:DC%2BD2cXis1ant74%3D
Lee J H, Paull T T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science, 2005, 308(5721): 551–554, 15790808, 10.1126/science.1108297, 1:CAS:528:DC%2BD2MXjtlOjt7k%3D
Hartley K O, Gell D, Smith G C, et al. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell, 1995, 82(5): 849–856, 7671312, 10.1016/0092-8674(95)90482-4, 1:CAS:528:DyaK2MXotFagsrc%3D
Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science, 1995, 268(5218): 1749–1753, 7792600, 10.1126/science.7792600, 1:CAS:528:DyaK2MXmsFCitrs%3D
Barlow C, Hirotsune S, Paylor R, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell, 1996, 86(1): 159–171, 8689683, 10.1016/S0092-8674(00)80086-0, 1:CAS:528:DyaK28XktlGnsbc%3D
Harper J W, Elledge S J. The DNA damage response: ten years after. Mol Cell, 2007, 28(5): 739–745, 18082599, 10.1016/j.molcel.2007.11.015, 1:CAS:528:DC%2BD1cXktVWisQ%3D%3D
Lavin M F, Kozlov S. ATM activation and DNA damage response. Cell Cycle, 2007, 6(8): 931–942, 17457059, 1:CAS:528:DC%2BD2sXos1Ggsr0%3D
Rai R, Peng G, Li K, et al. DNA damage response: the players, the network and the role in tumor suppression. Cancer Genomics Proteomics, 2007, 4(2): 99–106, 17804872, 1:CAS:528:DC%2BD2sXkvVehu7s%3D
Siliciano J D, Canman C E, Taya Y, et al. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev 1997, 11(24): 3471–3481, 9407038, 10.1101/gad.11.24.3471, 1:CAS:528:DyaK1cXivFOqsg%3D%3D
Banin S, Moyal L, Shieh S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science, 1998, 281(5383): 1674–1677, 9733514, 10.1126/science.281.5383.1674, 1:CAS:528:DyaK1cXmtVKitLY%3D
Khanna K K, Keating K E, Kozlov S, et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet, 1998, 20(4): 398–400, 9843217, 10.1038/3882, 1:CAS:528:DyaK1cXnslOls78%3D
Shieh S Y, Ikeda M, Taya Y, et al. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell, 1997, 91(3): 325–334, 9363941, 10.1016/S0092-8674(00)80416-X, 1:CAS:528:DyaK2sXnt1CltLY%3D
Corcoran C A, Huang Y, Sheikh M S. The p53 paddy wagon: COP1, Pirh2 and MDM2 are found resisting apoptosis and growth arrest. Cancer Biol Ther, 2004, 3(8): 721–725, 15280670, 1:CAS:528:DC%2BD2MXhslKrtL8%3D, 10.4161/cbt.3.8.1068
Dornan D, Wertz I, Shimizu H, et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature, 2004, 429(6987): 86–92, 15103385, 10.1038/nature02514, 1:CAS:528:DC%2BD2cXjs1Kiurk%3D
Marchler-Bauer A, Anderson J B, Derbyshire M K, et al. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res, 2007, 35 (Database issue): D237–240, 17135202, 10.1093/nar/gkl951, 1:CAS:528:DC%2BD2sXivFKntw%3D%3D
Krude T, Jackman M, Pines J, et al. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell, 1997, 88(1): 109–119, 9019396, 10.1016/S0092-8674(00)81863-2, 1:CAS:528:DyaK2sXltF2hsw%3D%3D
Shu H B, Takeuchi M, Goeddel D V. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci USA, 1996, 93(24): 13973–13978, 8943045, 10.1073/pnas.93.24.13973, 1:CAS:528:DyaK28Xnt1Gluro%3D
Ward I M, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem, 2001, 276(51): 47759–47762, 11673449, 1:CAS:528:DC%2BD38XkvFWj
Matsuda A, Suzuki Y, Honda G, et al. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene, 2003, 22(21): 3307–3318, 12761501, 10.1038/sj.onc.1206406, 1:CAS:528:DC%2BD3sXjvFaksLg%3D
Xu L G, Wang Y Y, Han K J, et al. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell, 2005, 19(6): 727–740, 16153868, 10.1016/j.molcel.2005.08.014, 1:CAS:528:DC%2BD2MXhtVyhtb%2FJ
Hsu I C, Tokiwa T, Bennett W, et al. p53 gene mutation and integrated hepatitis B viral DNA sequences in human liver cancer cell lines. Carcinogenesis, 1993, 14(5): 987–992, 8389256, 10.1093/carcin/14.5.987, 1:CAS:528:DyaK3sXkvFalsrk%3D
Ragot T, Vincent N, Chafey P, et al. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature, 1993, 361(6413): 647–650, 8437625, 10.1038/361647a0, 1:CAS:528:DyaK3sXhs1WjsLc%3D
Anderson S C, Johnson D E, Harris M P, et al. p53 gene therapy in a rat model of hepatocellular carcinoma: intra-arterial delivery of a recombinant adenovirus. Clin Cancer Res, 1998, 4(7): 1649–1659, 9676839, 1:CAS:528:DyaK1cXks1Kit7s%3D
Meplan C, Mann K, Hainaut P. Cadmium induces conformational modifications of wild-type p53 and suppresses p53 response to DNA damage in cultured cells. J Biol Chem, 1999, 274(44): 31663–31670, 10531375, 10.1074/jbc.274.44.31663, 1:CAS:528:DyaK1MXntFOjs7o%3D
Hendrikse A S, Hunter A J, Keraan M, et al. Effects of low dose irradiation on TK6 and U937 cells: induction of p53 and its role in cell-cycle delay and the adaptive response. Int J Radiat Biol, 2000, 76(1): 11–21, 10665953, 10.1080/095530000138961, 1:CAS:528:DC%2BD3cXjs1Omtrk%3D
Grob T J, Novak U, Maisse C, et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ, 2001, 8(12): 1213–1223, 11753569, 10.1038/sj.cdd.4400962, 1:CAS:528:DC%2BD38XmtVKksg%3D%3D
Walerych D, Kudla G, Gutkowska M, et al. Hsp90 chaperones wild-type p53 tumor suppressor protein. J Biol Chem, 2004, 279(47): 48836–48845, 15358769, 10.1074/jbc.M407601200, 1:CAS:528:DC%2BD2cXpslKjsr0%3D
Enokimura N, Shiraki K, Kawakita T, et al. Vitamin K analog (compound 5) induces apoptosis in human hepatocellular carcinoma independent of the caspase pathway. Anticancer Drugs, 2005, 16(8): 837–844, 16096431, 10.1097/01.cad.0000175583.78574.d7, 1:CAS:528:DC%2BD2MXntlSktL0%3D
Kastan M B, Zhan Q, el-Deiry W S, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell, 1992, 71(4): 587–597, 1423616, 10.1016/0092-8674(92)90593-2, 1:CAS:528:DyaK3sXjsFWksA%3D%3D
Eppenberger U, Kueng W, Eppenberger-Castori S, et al. Molecular factors determine primary and secondary therapy of breast carcinoma. Ther Umsch, 1997, 54(8): 451–456, 9381415, 1:STN:280:DyaK2svjt1agtg%3D%3D
Prives C. Signaling to p53: breaking the MDM2-p53 circuit. Cell, 1998, 95(1): 5–8, 9778240, 10.1016/S0092-8674(00)81774-2, 1:CAS:528:DyaK1cXmslWmu7k%3D
Seoane J, Le H V, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature, 2002, 419(6908): 729–734, 12384701, 10.1038/nature01119, 1:CAS:528:DC%2BD38XnvVyrt7g%3D
Chipuk J E, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science, 2004, 303(5660): 1010–1014, 14963330, 10.1126/science.1092734, 1:CAS:528:DC%2BD2cXhtlWnu7o%3D
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Basic Research Program of China (Grant No. 2006CB504301), National High Technology Research and Development Program of China (Grant No. 2006AA02A306), and National Natural Science Foundation of China (Grant Nos. 30630019 and 30570959).
Rights and permissions
About this article
Cite this article
Zhang, M., Wang, R., Wang, Y. et al. The CXXC finger 5 protein is required for DNA damage-induced p53 activation. SCI CHINA SER C 52, 528–538 (2009). https://doi.org/10.1007/s11427-009-0083-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-009-0083-7