Abstract
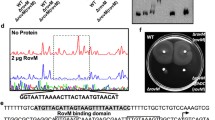
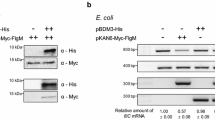
The flagella master regulatory gene flhDC of Yersinia pseudotuberculosis serotype III (YPIII) was mutated by deleting the middle region and replaced by a tetracycline resistant gene, and the subsequent mutant strain named YPIIIΔflhDC was obtained. Swimming assay showed that the swimming motility of the mutant strain was completely abolished. The promoter region of the flagella second-class regulatory gene fliA was fused with the lux box, and was conjugated with the mutant and the parent strains respectively for the first cross. LUCY assay result demonstrated that flhDC regulated the expression of fliA in YPIII as reported in E. coli. Biofilm formation of the mutant strain on abiotic and biotic surfaces was observed and quantified. The results showed that mutation of flhDC decreased biofilm formation on both abiotic and biotic surfaces, and abated the infection on Caenorhabdtis elegans. Our results suggest that mutation of the flagella master regulatory gene flhDC not only abolished the swimming motility, but also affected biofilm formation of YPIII on different surfaces. The new function of flhDC identified in this study provides a novel viewpoint for the control of bacterial biofilm formation.
Similar content being viewed by others
References
Wang S, Fleming R T, Westbrook E M, et al. Structure of the Escherichia coli FlhDC complex, a prokaryotic heteromeric regulator of transcription. J Mol Biol, 2006, 355: 798–808, 16337229, 10.1016/j.jmb.2005.11.020, 1:CAS:528:DC%2BD2MXhtlCrt73O
Macnab R M. Genetics and biogenesis of bacterial flagella. Annu Rev Genet, 1992, 26: 131–158, 1482109, 10.1146/annurev.ge.26.120192.001023, 1:CAS:528:DyaK3sXkt1Wgsb8%3D
Atkinson S, Chang C Y, Sockett R E, et al. Quorum sensing in Yersinia enterocolitica controls swimming and swarming motility. J Bacteriol, 2006, 188: 1451–1461, 16452428, 10.1128/JB.188.4.1451-1461.2006, 1:CAS:528:DC%2BD28Xhs1Gls70%3D
Prusß M, Liu X, Hendrickson W, et al. FlhD/flhC-regulated promoters analyzed by gene array and lacZ gene fusions. FEMS Microbiol Lett, 2001, 197: 91–97, 10.1016/S0378-1097(01)00092-1
Prusß M, Campbell J W, Van Dyk T K, et al. FlhD/FlhC is a regulator of anaerobic respiration and the Entner-Doudoroff pathway through induction of the methyl-accepting chemotaxis protein Aer. J Bacteriol, 2003, 185: 534–543, 10.1128/JB.185.2.534-543.2003, 1:CAS:528:DC%2BD3sXksl2ksg%3D%3D
Bleves S, Marenne M N, Detry G, et al. Up-regulation of the Yersinia enterocolitica Yop regulon by deletion of the flagellum master operon flhDC. J Bacteriol, 2002, 184: 3214–3223, 12029037, 10.1128/JB.184.12.3214-3223.2002, 1:CAS:528:DC%2BD38XksV2ht74%3D
Schmiel D H, Young G M, Miller V L. The Yersinia enterocolitica phospholipase gene yplA is part of the flagellar regulon. J Bacteriol, 2000,182: 2314–2320, 10735878, 10.1128/JB.182.8.2314-2320.2000, 1:CAS:528:DC%2BD3cXitlGgu7w%3D
Givskov M, Eberl L, Christiansen M, et al. Induction of phospholipase and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon FlhD. Mol Microbiol, 1995, 15: 445–454, 7783615, 10.1111/j.1365-2958.1995.tb02258.x, 1:CAS:528:DyaK2MXjvFejtrg%3D
Givaudan A, Lanois A. FlhDC, the flagellar master operon of Xenorhabdus nematophilus: requirement for motility, lipolysis, extracellular hemolysis, and full virulence in insects. J Bacteriol, 2000, 182: 107–115, 10613869, 1:CAS:528:DyaK1MXotFyjtL8%3D
Garbom S, Forsberg A K, Wolf-Watz H, et al. Identification of novel virulence-associated genes via genome analysis of hypothetical genes. Infect Immun, 2004, 72: 1333–1340., 14977936, 10.1128/IAI.72.3.1333-1340.2004, 1:CAS:528:DC%2BD2cXhvVChtLY%3D
McClean K H, Winson M K, Fish L, et al. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology, 1997, 143: 3703–3711, 9421896, 1:CAS:528:DyaK1cXnslOj, 10.1099/00221287-143-12-3703
Wang Y, Dai Y, Zhang Y, et al. Effects of quorum sensing autoinducer degradation gene on virulence and biofilm formation of Pseudomonas aeruginosa. Sci China Ser C-Life Sci, 2007, 50: 385–391, 10.1007/s11427-007-0044-y, 1:CAS:528:DC%2BD2sXosF2hs78%3D
Tan L, Darby C. A movable surface: Formation of Yersinia sp. biofilms on motile Caenorhabditis elegans. J Bacteriol, 2004, 186: 5087–5092, 15262945, 10.1128/JB.186.15.5087-5092.2004, 1:CAS:528:DC%2BD2cXmtFeqtrg%3D
Atkinson S, Throup J P, Stewart G S, et al. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol Microbiol, 1999, 33: 1267–1277, 10510240, 10.1046/j.1365-2958.1999.01578.x, 1:CAS:528:DyaK1MXmtlajsLw%3D
Joshua G W P, Karlyshev A V, Smith M P, et al. A Caenorhabditis elegans model of Yersinia infection: Biofilm formation on a biotic surface. Microbiology, 2003, 149: 3221–3229, 14600234, 10.1099/mic.0.26475-0, 1:CAS:528:DC%2BD3sXpsFagtLw%3D
Clutterbuck A L, Woods E J, Knottenbelt D C, et al. Biofilms and their relevance to veterinary medicine. Vet Microbiol, 2007, 121: 1–17, 17276630, 10.1016/j.vetmic.2006.12.029, 1:CAS:528:DC%2BD2sXisVCgtbw%3D
Simmons W L, Bolland J R, Daubenspeck J M, et al. A stochastic mechanism for biofilm formation by Mycoplasma pulmonis. J Bacteriol, 2007, 189: 1905–1913, 17142389, 10.1128/JB.01512-06, 1:CAS:528:DC%2BD2sXitl2hs7w%3D
Spoering A L, Gilmore M S. Quorum sensing and DNA release in bacterial biofilms. Curr Opin Microbiol, 2006, 9: 133–137, 16529982, 10.1016/j.mib.2006.02.004, 1:CAS:528:DC%2BD28XjtFagtLg%3D
Jefferson K K. What drives bacteria to produce a biofilm? FEMS Microbiol Lett, 2004, 236: 163–173, 15251193, 1:CAS:528:DC%2BD2cXls1ajtrc%3D
Pratt L A, Kotler R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol, 1998, 30: 285–293, 9791174, 10.1046/j.1365-2958.1998.01061.x, 1:CAS:528:DyaK1cXntFOrtrw%3D
O’Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol, 1998, 30: 295–304, 9791175, 10.1046/j.1365-2958.1998.01062.x, 1:CAS:528:DyaK1cXntFOrtr0%3D
Kalmokoff M, Lanthier P, Tremblay T L, et al. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J Bacteriol, 2006, 188: 4312–4320, 16740937, 10.1128/JB.01975-05, 1:CAS:528:DC%2BD28XmtV2hu74%3D
Lauriano C M, Ghosh C, Correa N E, et al. The sodium-driven flagellar motor controls exopolysaccharide expression in Vibrio cholerae. J Bacteriol, 2004,186: 4864–4874, 15262923, 10.1128/JB.186.15.4864-4874.2004, 1:CAS:528:DC%2BD2cXmtFeqsLo%3D
Klausen M, Heydorn A, Lambertsen L, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol, 2003, 48: 1511–1524, 12791135, 10.1046/j.1365-2958.2003.03525.x, 1:CAS:528:DC%2BD3sXkvFCmtLg%3D
Huber B, Riedel K, Hentzer M, et al. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology, 2001, 147: 2517–2528, 11535791, 1:CAS:528:DC%2BD3MXnt1Cgs7Y%3D
Suntharalingam P, Cvitkovitch D G. Quorum sensing in Streptococcal biofilm formation. Trends Microbiol, 2005, 13: 3–6, 15639624, 10.1016/j.tim.2004.11.009, 1:CAS:528:DC%2BD2MXhvF2nsg%3D%3D
Harris S J, Shih Y L, Bentley S D, et al. The hexA gene of Erwinia carotovora encodes a LysR homologue and regulates motility and the expression of multiple virulence determinants. Mol Microbiol, 1998, 28: 705–717, 9643539, 10.1046/j.1365-2958.1998.00825.x, 1:CAS:528:DyaK1cXjsFSgsrw%3D
Sperandio V, Torres A G, Kaper J B. Quorum sensing Escherichia coli regulators B and C (QseBC): A novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol, 2002, 43: 809–821, 11929534, 10.1046/j.1365-2958.2002.02803.x, 1:CAS:528:DC%2BD38XitlWgsrw%3D
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 30570020)
Rights and permissions
About this article
Cite this article
Wang, Y., Ding, L., Hu, Y. et al. The flhDC gene affects motility and biofilm formation in Yersinia pseudotuberculosis. SCI CHINA SER C 50, 814–821 (2007). https://doi.org/10.1007/s11427-007-0101-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-007-0101-6