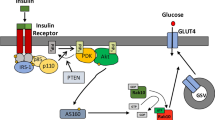
Abstract
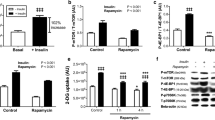
L6 skeletal muscle myoblasts stably overexpressing glucose transporter GLUT1 or GLUT4 with exofacial myc-epitope tags were characterized for their response to insulin. In clonally selected cultures, 2-deoxyglucose uptake into L6-GLUT1myc myoblasts and myotubes was linear within the time of study. In L6-GLUT1myc and L6-GLUT4myc myoblasts, 100 nmol/L insulin treatment increased the GLUT1 content of the plasma membrane by 1.58±0.01 fold and the GLUT4 content 1.96±0.11 fold, as well as the 2-deoxyglucose uptake 1.53±0.09 and 1.86±0.17 fold respectively, all by a wortmannin-inhibitable manner. The phosphorylation of Akt in these two cell lines was increased by insulin. L6-GLUT1myc myoblasts showed a dose-dependent stimulation of glucose uptake by insulin, with unaltered sensitivity and maximal responsiveness compared with wild type cells. By contrast, the improved insulin responsiveness and sensitivity of glucose uptake were observed in L6-GLUT4myc myoblasts. Earlier studies indicated that forskolin might affect insulin-stimulated GLUT4 translocation. A 65% decrease of insulin-stimulated 2-deoxyglucose uptake in GLUT4myc cells was not due to an effect on GLUT4 mobilization to the plasma membrane, but instead on direct inhibition of GLUT4. Forskolin and dipyridamole are more potent inhibitors of GLUT4 than GLUT1. Alternatively, pentobarbital inhibits GLUT1 more than GLUT4. The use of these inhibitors confirmed that the overexpressed GLUT1 or GLUT4 are the major functional glucose transporters in unstimulated and insulin-stimulated L6 myoblasts. Therefore, L6-GLUT1myc and L6-GLUT4myc cells provide a platform to screen compounds that may have differential effects on GLUT isoform activity or may influence GLUT isoform mobilization to the cell surface of muscle cells.
Similar content being viewed by others
References
Mitsumoto Y, Liu Z, Klip A. A long-lasting vitamin C derivative, ascorbic acid 2-phosphate, increases myogenin gene expression and promotes differentiation in L6 muscle cells. Biochem Biophys Res Commun, 1994, 199(1): 394–402, 8123041, 10.1006/bbrc.1994.1242, 1:CAS:528:DyaK2cXitFGrs7Y%3D
Marsh B J, Alm R A, McIntosh S R, et al. Molecular regulation of GLUT-4 targeting in 3T3-L1 adipocytes. J Cell Biol, 1995, 130: 1081–1091
Sargeant R, Mitsumoto Y, Sarabia V, et al. Hormonal regulation of glucose transporters in muscle cells in culture. J Endocrinol Invest, 1993, 16(2): 147–162
Mitsumoto Y, Burdett E, Grant A, et al. Differential expression of the GLUT1 and GLUT4 glucose transporters during differentiation of L6 muscle cells. Biochem Biophys Res Commun, 1991, 175(2): 652–659
Wang Q, Khayat Z, Kishi K, et al. GLUT4 translocation by insulin in intact muscle cells: Detection by a fast and quantitative assay. FEBS Lett, 1998, 427(2): 193–197
Rudich A, Konrad D, Torok D, et al. Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia, 2003, 46(5): 649–658
Ueyama A, Yaworsky K L, Wang Q, et al. GLUT4myc ectopic expression in L6 myoblasts generates a GLUT-4-specific pool conferring insulin sensitivity. Am J Physiol, 1999, 277(3 Pt 1): E572–578
Hellwig B, Joost H G. Differentiation of erythrocyte-(GLUT1), liver-(GLUT2), and adipocyte-type (GLUT4) glucose transporters by binding of the inhibitory ligands cytochalasin B, forskolin, dipyridamole, and isobutylmethylxanthine. Mol Pharmacol, 1991, 40(3): 383–389
Haspel H C, Stephenson K N, Davies-Hill T, et al. Effects of barbiturates on facilitative glucose transporters are pharmacologically specific and isoform selective. J Membr Biol, 1999, 169(1): 45–53
Murata H, Hruz P W, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem, 2000, 275(27): 20251–20254
Kanai F, Nishioka Y, Hayashi H, et al. Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J Biol Chem, 1993, 268(19): 14523–14526
Kishi K, Muromoto N, Nakaya Y, et al. Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes, 1998, 47(4): 550–558
Somwar R, Niu W, Kim D Y, et al. Differential effects of phosphatidylinositol 3-kinase inhibition on intracellular signals regulating GLUT4 translocation and glucose transport. J Biol Chem, 2001, 276(49): 46079–46087
Li D, Randhawa V K, Patel N, et al. Hyperosmolarity reduces GLUT4 endocytosis and increases its exocytosis from a VAMP2-independent pool in L6 muscle cells. J Biol Chem, 2001, 276(25): 22883–22891
Huang C, Somwar R, Patel N, et al. Sustained exposure of L6 myotubes to high glucose and insulin decreases insulin-stimulated GLUT4 translocation but upregulates GLUT4 activity. Diabetes, 2002, 51(7): 2090–2098
Wang Q, Somwar R, Bilan P J, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol, 1999, 19: 4008–4018
Mitsumoto Y and Klip A. Development regulation of the subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J Biol Chem, 1992, 267: 4957–4962
Bilan PJ, Mitsumoto Y, Maher F, et al. Detection of the GLUT3 facilitative glucose transporter in rat L6 muscle cells: Regulation by cellular differentiation, insulin and insulin-like growth factor-I. Biochem Biophys Res Commun, 1992, 186(2): 1129–1137
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 30570912), the National Natural Science Foundation of China (China-Canada Joint Health Research) (Grant No. 30611120532), the Tianjin Municipal Science and Technology Commission, China (Grant Nos. 07JCZDJC07900 and 06YFGPSH03300), and the Foundation of Tianjin Education Bureau, China (Grant No. 20040106)
Rights and permissions
About this article
Cite this article
Niu, W., Bilan, P.J., Hayashi, M. et al. Insulin sensitivity and inhibition by forskolin, dipyridamole and pentobarbital of glucose transport in three L6 muscle cell lines. SCI CHINA SER C 50, 739–747 (2007). https://doi.org/10.1007/s11427-007-0088-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-007-0088-z