Abstract
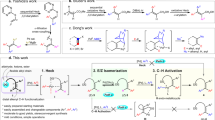
The highly diastereoselective dicarbofunctionalization of substituted olefins still remains a daunting challenge in synthetic chemistry. Herein, we report a Pd-catalyzed diastereoselective 1,1-diarylation of 1,1-diarylethylene with chelation group free to enable the modular synthesis of smart material candidates, 1,1,2,2-tetraarylethanes (TAEs), which represent the first protocol by assembling four different aryl groups into ethane motif. Preliminary mechanistic experiments suggest that the crucial Pd-H species generated in situ do not disengage from the alkene intermediate during the reaction.

Similar content being viewed by others
References
Oxtoby LJ, Vasquez AM, Kang T, Li Z-Q, Engle KM. Metal-Mediated and Catalyzed Difunctionalization of Unsaturated Organics. Comprehensive Organometallic Chemistry IV. Volume 13: Applications II. d- and f-Block Metal Complexes in Organic Synthesis-part 2. Amsterdam: Elsevier, 2022. 132–193
Wickham LM, Giri R. Acc Chem Res, 2021, 54: 3415–3437
Wang H, Koh MJ. Cell Rep Phys Sci, 2022, 3: 100901–100909
Wang Z, Bai X, Li B. Chin J Chem, 2019, 37: 1174–1180
Zhu S, Zhao X, Li H, Chu L. Chem Soc Rev, 2021, 50: 10836–10856
Luo Y, Xu C, Zhang X. Chin J Chem, 2020, 38: 1371–1394
Qi X, Diao T. ACS Catal, 2020, 10: 8542–8556
Li ZL, Fang GC, Gu QS, Liu XY. Chem Soc Rev, 2020, 49: 32–48
Ping Y, Song H, Kong W. Chin J Org Chem, 2022, 42: 3302–3321
Liao L, Jana R, Urkalan KB, Sigman MS. J Am Chem Soc, 2011, 133: 5784–5787
Zhang L, Lovinger GJ, Edelstein EK, Szymaniak AA, Chierchia MP, Morken JP. Science, 2016, 351: 70–74
Dhungana RK, Aryal V, Niroula D, Sapkota RR, Lakomy MG, Giri R. Angew Chem Int Ed, 2021, 60: 19092–19096
Zhang WB, Chen G, Shi SL. J Am Chem Soc, 2022, 144: 130–136
Liu Z, Zeng T, Yang KS, Engle KM. J Am Chem Soc, 2016, 138: 15122–15125
Shrestha B, Basnet P, Dhungana RK, Kc S, Thapa S, Sears JM, Giri R. J Am Chem Soc, 2017, 139: 10653–10656
Gao P, Chen LA, Brown MK. J Am Chem Soc, 2018, 140: 10653–10657
Myhill JA, Wilhelmsen CA, Zhang L, Morken JP. J Am Chem Soc, 2018, 140: 15181–15185
Thapa S, Dhungana RK, Magar RT, Shrestha B, Kc S, Giri R. Chem Sci, 2018, 9: 904–909
Basnet P, Kc S, Dhungana RK, Shrestha B, Boyle TJ, Giri R. J Am Chem Soc, 2018, 140: 15586–15590
Derosa J, Kleinmans R, Tran VT, Karunananda MK, Wisniewski SR, Eastgate MD, Engle KM. J Am Chem Soc, 2018, 140: 17878–17883
Anthony D, Lin Q, Baudet J, Diao T. Angew Chem Int Ed, 2019, 58: 3198–3202
Zhang Y, Chen G, Zhao D. Chem Sci, 2019, 10: 7952–7957
Derosa J, Kang T, Tran VT, Wisniewski SR, Karunananda MK, Jankins TC, Xu KL, Engle KM. Angew Chem Int Ed, 2020, 59: 1201–1205
Kleinmans R, Apolinar O, Derosa J, Karunananda MK, Li ZQ, Tran VT, Wisniewski SR, Engle KM. Org Lett, 2021, 23: 5311–5316
Apolinar O, Kang T, Alturaifi TM, Bedekar PG, Rubel CZ, Derosa J, Sanchez BB, Wong QN, Sturgell EJ, Chen JS, Wisniewski SR, Liu P, Engle KM. J Am Chem Soc, 2022, 144: 19337–19343
Dong Z, Tang Q, Xu C, Chen L, Ji H, Zhou S, Song L, Chen L. Angew Chem Int Ed, 2023, 62: e202218286
Saini V, Sigman MS. J Am Chem Soc, 2012, 134: 11372–11375
Jeon J, Ryu H, Lee C, Cho D, Baik MH, Hong S. J Am Chem Soc, 2019, 141: 10048–10059
Werner EW, Urkalan KB, Sigman MS. Org Lett, 2010, 12: 2848–2851
Urkalan K, Sigman M. Angew Chem Int Ed, 2009, 48: 3146–3149
Saini V, Liao L, Wang Q, Jana R, Sigman MS. Org Lett, 2013, 15: 5008–5011
Orlandi M, Hilton MJ, Yamamoto E, Toste FD, Sigman MS. J Am Chem Soc, 2017, 139: 12688–12695
Yamamoto E, Hilton MJ, Orlandi M, Saini V, Toste FD, Sigman MS. J Am Chem Soc, 2016, 138: 15877–15880
Shao H, Zhao Y, Wang S, Chen R, Zhou JS, Wu X. Org Lett, 2022, 24: 6520–6524
Li Z, Wu D, Ding C, Yin G. CCS Chem, 2021, 3: 576–582
Li Y, Wei H, Wu D, Li Z, Wang W, Yin G. ACS Catal, 2020, 10: 4888–4894
Sun C, Li Y, Yin G. Angew Chem Int Ed, 2022, 61: e202209076
Sun S, Talavera L, Spieß P, Day CS, Martin R. Angew Chem Int Ed, 2021, 60: 11740–11744
Li Y, Pang H, Wu D, Li Z, Wang W, Wei H, Fu Y, Yin G. Angew Chem Int Ed, 2019, 58: 8872–8876
Wang W, Ding C, Yin G. Nat Catal, 2020, 3: 951–958
Satterfield AD, Kubota A, Sanford MS. Org Lett, 2011, 13: 1076–1079
Kalyani D, Satterfield AD, Sanford MS. J Am Chem Soc, 2010, 132: 8419–8427
Kalyani D, Sanford MS. J Am Chem Soc, 2008, 130: 2150–2151
Li Y, Li Y, Shi H, Wei H, Li H, Funes-Ardoiz I, Yin G. Science, 2022, 376: 749–753
Li Z, Shi H, Chen X, Peng L, Li Y, Yin G. J Am Chem Soc, 2023, 145: 13603–13614
Bergmann AM, Dorn SK, Smith KB, Logan KM, Brown MK. Angew Chem Int Ed, 2019, 58: 1719–1723
Xi Y, Huang W, Wang C, Ding H, Xia T, Wu L, Fang K, Qu J, Chen Y. J Am Chem Soc, 2022, 144: 8389–8398
Xia T, Xi Y, Ding H, Zhang Y, Fang K, Wu X, Qu J, Chen Y. Chem Commun, 2022, 58: 9282–9285
Wu X, Turlik A, Luan B, He F, Qu J, Houk KN, Chen Y. Angew Chem Int Ed, 2022, 61: e202207536
Wu X, Luan B, Zhao W, He F, Wu X, Qu J, Chen Y. Angew Chem Int Ed, 2022, 61: e202111598
Teng S, Chi YR, Zhou JS. Angew Chem Int Ed, 2021, 60: 4491–4495
Teng S, Jiao Z, Chi YR, Zhou JS. Angew Chem Int Ed, 2020, 59: 2246–2250
Wu L, Wang F, Chen P, Liu G. J Am Chem Soc, 2019, 141: 1887–1892
Wu D, Wu L, Chen P, Liu G. Chin J Chem, 2022, 40: 1699–1704
Wang L, Wang C. Org Lett, 2020, 22: 8829–8835
Wang H, Liu CF, Martin RT, Gutierrez O, Koh MJ. Nat Chem, 2022, 14: 188–195
Wang H, Liu CF, Tan TD, Khoo KRB, Koh MJ. ACS Catal, 2022, 12: 724–732
Liu CF, Wang ZC, Luo X, Lu J, Ko CHM, Shi SL, Koh MJ. Nat Catal, 2022, 5: 934–942
Li Y, Wu D, Cheng H, Yin G. Angew Chem Int Ed, 2020, 59: 7990–8003
Zhang M, Ji Y, Zhang C. Chin J Chem, 2022, 40: 1608–1622
Belal M, Li Z, Lu X, Yin G. Sci China Chem, 2021, 64: 513–533
Dhungana RK, Sapkota RR, Niroula D, Giri R. Chem Sci, 2020, 11: 9757–9774
Sommer H, Juliá-Hernández F, Martin R, Marek I. ACS Cent Sci, 2018, 4: 153–165
Basnet P, Dhungana RK, Thapa S, Shrestha B, Kc S, Sears JM, Giri R. J Am Chem Soc, 2018, 140: 7782–7786
Li W, Boon JK, Zhao Y. Chem Sci, 2018, 9: 600–607
Ding C, Ren Y, Sun C, Long J, Yin G. J Am Chem Soc, 2021, 143: 20027–20034
Zhu D, Xu W, Pu M, Wu YD, Chi YR, Zhou JS. Org Lett, 2021, 23: 7064–7068
Pang H, Wu D, Yin G. Chin J Org Chem, 2021, 41: 849
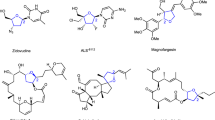
Zhang H, Zheng X, Xie N, He Z, Liu J, Leung NLC, Niu Y, Huang X, Wong KS, Kwok RTK, Sung HHY, Williams ID, Qin A, Lam JWY, Tang BZ. J Am Chem Soc, 2017, 139: 16264–16272
Li Z, Yin S, Tan G, Zhao S, Shi Z, Jing B, Zhai L, Tan Y. Colloid Polym Sci, 2016, 294: 1943–1958
Tojo Y, Arakwa Y, Watanabe J, Konishi G. Polym Chem, 2013, 4: 3807–3812
Sumi K, Niko Y, Tokumaru K, Konishi G. Chem Commun, 2013, 49: 3893–3895
Li SMK. Process for Preparing Tetraphenolic Compounds. US Patent, US19915012016, 1991-04-30
Kimura Y, Noda K, Ogiwara A. Method for Producing Tetrakis(4-Hydroxyphenyl) Ethane Compound. JP Patent, JP2005263676, 2005-09-29
Takemura K. Method for Producing Tetrakisphenolethanes. JP Patent, JP2016199488, 2016-12-01
Li Y, Kijima T, Izumi T. J Organomet Chem, 2003, 687: 12–15
Pan FF, Guo P, Huang X, Shu XZ. Synthesis, 2021, 53: 3094–3100
Pratt EF, Suskind SP. J Org Chem, 1963, 28: 638–642
Cao D, Li CC, Zeng H, Peng Y, Li CJ. Nat Commun, 2021, 12: 3729–3736
Zhao Z, Zhang H, Lam JWY, Tang BZ. Angew Chem Int Ed, 2020, 59: 9888–9907
Ju B, Chen S, Kong W. Org Lett, 2019, 21: 9343–9347
Johnson J, Rovis T. Angew Chem Int Ed, 2008, 47: 840–871
Derosa J, Kleinmans R, Tran VT, Karunananda MK, Wisniewski SR, Eastgate MD, Engle KM. J Am Chem Soc, 2018, 140: 17878–17883
Wu Z, Fatuzzo N, Dong G. J Am Chem Soc, 2020, 142: 2715–2720
Mei TS, Patel HH, Sigman MS. Nature, 2014, 508: 340–344
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22171079), the Natural Science Foundation of Shanghai (21ZR1480400), the Shanghai Rising-Star Program (20QA1402300), the Shanghai Municipal Science and Technology Major Project (2018SHZDZX03), the Program of Introducing Talents of Discipline to Universities (B16017), the Fundamental Research Funds for the Central Universities. Y.C. thanks Prof. Weijun Zhao (East China University of Science and Technology) for insightful discussion. The authors thank the Analysis and Testing Center of East China University of Science and Technology for help with NMR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at chem.scichina.com and link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2023_1780_MOESM1_ESM.pdf
Pd-catalyzed diastereoselective 1,1-diarylation of 1,1-disubstituted alkenes enabling the modular synthesis of 1,1,2,2-tetraarylethanes
Rights and permissions
About this article
Cite this article
Zhang, C., Xi, Y., Qu, J. et al. Pd-catalyzed diastereoselective 1,1-diarylation of 1,1-disubstituted alkenes enabling the modular synthesis of 1,1,2,2-tetraarylethanes. Sci. China Chem. 66, 3539–3545 (2023). https://doi.org/10.1007/s11426-023-1780-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1780-2