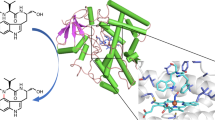
Abstract
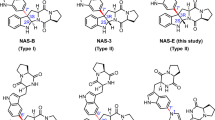
Indole alkaloids have attracted considerable attention from synthetic chemists and biochemists for their structural diversity and important biological activities. Compared with traditional organic synthesis methods, the strategy of using cytochrome P450s’ extraordinary abilities to selectively activate carbon-hydrogen bonds to assist in the synthesis of various indole alkaloids has the characteristics of short synthetic route, mild conditions and high atomic economy. Here, we utilized P450 monooxygenases HinD and TleB to synthesize a novel 6/5/8 tricyclic product from (S)-N-((S)-1-(4-fluoro-1H-indol-3-yl)-3-hydroxypropan-2-yl)-2-mercapto-3-methylbutanamide through the substrate structure-directed strategy. TleB was more effective in catalyzing C–S coupling, and was used to synthesize a series of 6/5/8 tricyclic indololactam derivatives to provide drug candidates. Interestingly, the S–S coupling product was observed in HinD catalysis, which was a minor product in the wild-type TleB catalysis. With the help of protein engineering, we accurately regulated the catalytic flow and reversed the selectivity of TleB to obtain the S–S coupling product. At the same time, the reaction mechanism was reasonably speculated by means of site blocking and protein-substrate complex analysis.

Similar content being viewed by others
References
Newman DJ. Natl Sci Rev, 2022, 9: nwac206
Newman DJ, Cragg GM. J Nat Prod, 2020, 83: 770–803
Rodrigues T, Reker D, Schneider P, Schneider G. Nat Chem, 2016, 8: 531–541
Zhou B, Yue JM. Natl Sci Rev, 2022, 9: nwac112
Godula K, Sames D. Science, 2006, 312: 67–72
Labinger JA, Bercaw JE. Nature, 2002, 417: 507–514
Yamaguchi J, Yamaguchi AD, Itami K. Angew Chem Int Ed, 2012, 51: 8960–9009
Barry SM, Kers JA, Johnson EG, Song L, Aston PR, Patel B, Krasnoff SB, Crane BR, Gibson DM, Loria R, Challis GL. Nat Chem Biol, 2012, 8: 814–816
Devine AJ, Parnell AE, Back CR, Lees NR, Johns ST, Zulkepli AZ, Barringer R, Zorn K, Stach JEM, Crump MP, Hayes MA, van der Kamp MW, Race PR, Willis CL. Angew Chem Int Ed, 2023, 62: e202213053
Grandner JM, Cacho RA, Tang Y, Houk KN. ACS Catal, 2016, 6: 4506–4511
Li S, Tietz DR, Rutaganira FU, Kells PM, Anzai Y, Kato F, Pochapsky TC, Sherman DH, Podust LM. J Biol Chem, 2012, 287: 37880–37890
Lin Z, Xue Y, Liang XW, Wang J, Lin S, Tao J, You SL, Liu W. Angew Chem Int Ed, 2021, 60: 8401–8405
Sherman DH, Li S, Yermalitskaya LV, Kim Y, Smith JA, Waterman MR, Podust LM. J Biol Chem, 2006, 281: 26289–26297
Wang H, Zou Y, Li M, Tang Z, Wang J, Tian Z, Strassner N, Yang Q, Zheng Q, Guo Y, Liu W, Pan L, Houk KN. Nat Chem, 2023, 15: 177–184
Long Y, Zheng S, Feng Y, Yang Z, Xu X, Song H. ACS Catal, 2022, 12: 9857–9863
Lima LM, Silva BNM, Barbosa G, Barreiro EJ. Eur J Med Chem, 2020, 208: 112829
Ye W, Liu T, Liu Y, Li M, Wang S, Li S, Zhang W. Bioresource Tech, 2023, 377: 128905
Xiong K, Xue S, Guo H, Dai Y, Ji C, Dong L, Zhang S. Crit Rev Food Sci Nutr, 2023, 1–12
Shi Y, Jiang Z, Hu X, Hu X, Gu R, Jiang B, Zuo L, Li X, Sun H, Zhang C, Wang L, Wu L, Hong B. Angew Chem Int Ed, 2021, 60: 15399–15404
Morita I, Mori T, Mitsuhashi T, Hoshino S, Taniguchi Y, Kikuchi T, Nagae K, Nasu N, Fujita M, Ohwada T, Abe I. Angew Chem Int Ed, 2020, 59: 3988–3993
LaValle CR, Bravo-Altamirano K, Giridhar KV, Chen J, Sharlow E, Lazo JS, Wipf P, Wang QJ. BMC Chem Biol, 2010, 10: 5
Chen K, Arnold FH. J Am Chem Soc, 2020, 142: 6891–6895
Cui L, Cui A, Li Q, Yang L, Liu H, Shao W, Feng Y. ACS Catal, 2022, 12: 13703–13714
Engström K, Nyhlén J, Sandström AG, Bäckvall JE. J Am Chem Soc, 2010, 132: 7038–7042
Prakinee K, Phintha A, Visitsatthawong S, Lawan N, Sucharitakul J, Kantiwiriyawanitch C, Damborsky J, Chitnumsub P, van Pée KH, Chaiyen P. Nat Catal, 2022, 5: 534–544
Hong W, Swann WA, Yadav V, Li CW. ACS Catal, 2022, 12: 7643–7654
Schelhaas M, Waldmann H. Angew Chem Int Ed Engl, 1996, 35: 2056–2083
Wang W, Wan H, Du G, Dai B, He L. OrgLett, 2019, 21: 3496–3500
Curran MP, Keating GM. Drugs, 2005, 65: 2009–2035
Fan M, Huang Y, Zhu X, Zheng J, Du M. J Drug Target, 2023, 1–16
He F, Mori T, Morita I, Nakamura H, Alblova M, Hoshino S, Awakawa T, Abe I. Nat Chem Biol, 2019, 15: 1206–1213
Kabsch W. Acta Crystlogr D Biol Crystlogr, 2010, 66: 125–132
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. J Appl Crystlogr, 2007, 40: 658–674
Emsley P, Lohkamp B, Scott WG, Cowtan K. Acta Crystlogr D Biol Crystlogr, 2010, 66: 486–501
Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Acta Crystlogr D Biol Crystlogr, 2012, 68: 352–367
Liebschner D, Afonine PV, Baker ML, Bunkóczi G, Chen VB, Croll TI, Hintze B, Hung LW, Jain S, McCoy AJ, Moriarty NW, Oeffner RD, Poon BK, Prisant MG, Read RJ, Richardson JS, Richardson DC, Sammito MD, Sobolev OV, Stockwell DH, Terwilliger TC, Urzhumtsev AG, Videau LL, Williams CJ, Adams PD. Acta Crystlogr D Struct Biol, 2019, 75: 861–877
Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Proc Natl Acad Sci USA, 2001, 98: 10037–10041
Sun H, Keefer CE, Scott DO. DrugMetab Lett., 2011, 5: 232–242
Smith BR, Eastman CM, Njardarson JT. J Med Chem, 2014, 57: 9764–9773
Almond-Thynne J, Blakemore DC, Pryde DC, Spivey AC. Chem Sci, 2017, 8: 40–62
Akondi KB, Muttenthaler M, Dutertre S, Kaas Q, Craik DJ, Lewis RJ, Alewood PF. Chem Rev, 2014, 114: 5815–5847
Góngora-Benítez M, Tulla-Puche J, Albericio F. Chem Rev, 2014, 114: 901–926
Guo J, Zha J, Zhang T, Ding CH, Tan Q, Xu B. Org Lett, 2021, 23: 3167–3172
Chakraborty A, Albericio F, de la Torre BG. J Org Chem, 2022, 87: 708–712
Dou Y, Huang X, Wang H, Yang L, Li H, Yuan B, Yang G. Green Chem, 2017, 19: 2491–2495
Huang P, Wang P, Tang S, Fu Z, Lei A. Angew Chem Int Ed, 2018, 57: 8115–8119
Laps S, Atamleh F, Kamnesky G, Sun H, Brik A. Nat Commun, 2021, 12: 870
Landeta C, Boyd D, Beckwith J. Nat Microbiol, 2018, 3: 270–280
Liu H, Fan J, Zhang P, Hu Y, Liu X, Li SM, Yin WB. Chem Sci, 2021, 12: 4132–4138
Wang Z, Diao W, Wu P, Li J, Fu Y, Guo Z, Cao Z, Shaik S, Wang B. J Am Chem Soc, 2023, 145: 7252–7267
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0903200 to H.S.), Wuhan University, Undergraduate Training Programs for Innovation and Entrepreneurship of Wuhan University to Y.L., the Science and Technology Commission of Shanghai, and the National Natural Science Foundation of China (31900033, 21ZR1433700). The authors thank staff from BL19U1 beamline at Shanghai Synchrotron Radiation Facility (SSRF), China, for their assistance in X-ray crystal data collection.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information
The supporting information is available online at chem.scichina.com and link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2023_1755_MOESM1_ESM.docx
Regulation of P450 TleB Catalytic Flow for the Synthesis of Sulfur-Containing Indole Alkaloids by Substrate Structure-Directed Strategy and Protein Engineering, approximately 27.9 MB.
Rights and permissions
About this article
Cite this article
Ge, X., Long, Y., Wang, J. et al. Regulation of P450 TleB catalytic flow for the synthesis of sulfur-containing indole alkaloids by substrate structure-directed strategy and protein engineering. Sci. China Chem. 66, 3232–3241 (2023). https://doi.org/10.1007/s11426-023-1755-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1755-4