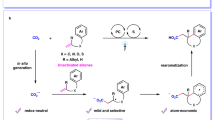
Abstract
A green method for synthesis of nitriles from aldehydes and ammonium salts under air is developed under extremely mild conditions, i.e., 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) as a photocatalyst, 2,2,6,6-tetrametylpiperidine-1-oxyl (TEMPO) as a cocatalyst, and oxygen (ambient air) as the terminal oxidant, visible light irradiation of substrate solutions, producing the desired nitriles with excellent yields. The reaction involves two distinct transformations, imine formation between an aldehyde and an ammonium salt and photocatalytic oxidation of the formed imine by air to a nitrile.

Similar content being viewed by others
References
Miller JS, Manson JL. Acc Chem Res, 2001, 34: 563–570
Fleming FF. Nat Prod Rep, 1999, 16: 597–606
Fleming FF, Yao L, Ravikumar PC, Funk L, Shook BC. J Med Chem, 2010, 53: 7902–7917
Murphy ST, Case HL, Ellsworth E, Hagen S, Huband M, Joannides T, Limberakis C, Marotti KR, Ottolini AM, Rauckhorst M, Starr J, Stier M, Taylor C, Zhu T, Blaser A, Denny WA, Lu GL, Smaill JB, Rivault F. Bioorg Med Chem Lett, 2007, 17: 2150–2155
Rappoport Z. Chemistry of the Cyano Group. Hoboken: John Wiley & Sons, 1970
Finholt AE, Jacobson EC, Ogard AE, Thompson P. J Am Chem Soc, 1955, 77: 4163
Gould F, Johnson G, Ferris A. J Org Chem, 1960, 25: 1658–1660
Michelin RA, Mozzon M, Bertani R. Coord Chem Rev, 1996, 147: 299–338
Polshettiwar V, Varma RS. Chem Eur J, 2009, 15: 1582–1586
Allen CL, Williams JMJ. Chem Soc Rev, 2011, 40: 3405–3415
Schmid TE, Gómez-Herrera A, Songis O, Sneddon D, Révolte A, Nahra F, Cazin CSJ. Catal Sci Technol, 2015, 5: 2865–2868
Raj J, Singh N, Prasad S, Seth A, Bhalla T. Acta Microbiologica Immunologica Hungarica, 2007, 54: 79–88
Movassaghi M, Hill MD. Nat Protoc, 2007, 2: 2018–2023
Das B, Reddy C, Kumar D, Krishnaiah M, Narender R. Synlett, 2010, 3: 391–394
Yang J, Karver MR, Li W, Sahu S, Devaraj NK. Angew Chem Int Ed, 2012, 51: 5222–5225
Sandmeyer T. Ber Dtsch Chem Ges, 1884, 17: 1633–1635
Hodgson HH. Chem Rev, 1947, 40: 251–277
Kim DW, Song CE, Chi DY. J Org Chem, 2003, 68: 4281–4285
Beletskaya IP, Sigeev AS, Peregudov AS, Petrovskii PV. J Organomet Chem, 2004, 689: 3810–3812
Rosenmund KW, Struck E. Ber dtsch Chem Ges A B, 1919, 52: 1749–1756
von Braun J, Manz G. Justus Liebigs Ann Chem, 1931, 488: 111–126
Patil RD, Gupta MK. Adv Synth Catal, 2020, 362: 3987–4009
Achard T, Egly J, Sigrist M, Maisse-François A, Bellemin-Laponnaz S. Chem Eur J, 2019, 25: 13271–13274
Kim J, Golime G, Kim HY, Oh K. Asian J Org Chem, 2019, 8: 1674–1679
Jia X, Ma J, Xia F, Gao M, Gao J, Xu J. Nat Commun, 2019, 10: 2338
Gan L, Jia X, Fang H, Liu G, Huang Z. ChemCatChem, 2020, 12: 3661–3665
Yang SH, Chang S. Org Lett, 2001, 3: 4209–4211
Yan G, Zhang Y, Wang J. Adv Synth Catal, 2017, 359: 4068–4105
Wang H, Dong Y, Zheng C, Sandoval CA, Wang X, Makha M, Li Y. Chem, 2018, 4: 2883–2893
Plass C, Hinzmann A, Terhorst M, Brauer W, Oike K, Yavuzer H, Asano Y, Vorholt AJ, Betke T, Gröger H. ACS Catal, 2019, 9: 5198–5203
Wang Y, Furukawa S, Yan N. ACS Catal, 2019, 9: 6681–6691
Wang Y, Furukawa S, Zhang Z, Torrente-Murciano L, Khan SA, Yan N. Catal Sci Technol, 2019, 9: 86–96
Hyodo K, Togashi K, Oishi N, Hasegawa G, Uchida K. Org Lett, 2017, 19: 3005–3008
Preger Y, Root TW, Stahl SS. ACS Omega, 2018, 3: 6091–6096
Xian C, He J, He Y, Nie J, Yuan Z, Sun J, Martens WN, Qin J, Zhu HY, Zhang Z. J Am Chem Soc, 2022, 144: 23321–23331
Ge JJ, Yao CZ, Wang MM, Zheng HX, Kang YB, Li Y. Org Lett, 2016, 18: 228–231
Wang H, Xu D, Guan E, Wang L, Zhang J, Wang C, Wang S, Xu H, Meng X, Yang B, Gates BC, Xiao FS. ACS Catal, 2020, 10: 6299–6308
Wang Y, Furukawa S, Fu X, Yan N. ACS Catal, 2020, 10: 311–335
Chen X, Song S, Li H, Gözaydιn G, Yan N. Acc Chem Res, 2021, 54: 1711–1722
Wu X, Luo N, Xie S, Zhang H, Zhang Q, Wang F, Wang Y. Chem Soc Rev, 2020, 49: 6198–6223
Rajender Reddy K, Uma Maheswari C, Venkateshwar M, Prashanthi S, Lakshmi Kantam M. Tetrahedron Lett, 2009, 50: 2050–2053
Waldvogel SR. Synthesis, 2010, 5: 892
Pradal A, Evano G. Chem Commun, 2014, 50: 11907–11910
Oishi T, Yamaguchi K, Mizuno N. Angew Chem Int Ed, 2009, 48: 6286–6288
Oishi T, Yamaguchi K, Mizuno N. Top Catal, 2010, 53: 479–486
Dornan LM, Cao Q, Flanagan JCA, Crawford JJ, Cook MJ, Muldoon MJ. Chem Commun, 2013, 49: 6030–6032
Dighe SU, Chowdhury D, Batra S. Adv Synth Catal, 2014, 356: 3892–3896
Kelly CB, Lambert KM, Mercadante MA, Ovian JM, Bailey WF, Leadbeater NE. Angew Chem Int Ed, 2015, 54: 4241–4245
Noh JH, Kim J. J Org Chem, 2015, 80: 11624–11628
Tian X, Ren YL, Ren F, Cheng X, Zhao S, Wang J. Synlett, 2018, 29: 2444–2448
Hua M, Song J, Huang X, Liu H, Fan H, Wang W, He Z, Liu Z, Han B. Angew Chem Int Ed, 2021, 60: 21479–21485
Prier CK, Rankic DA, MacMillan DWC. Chem Rev, 2013, 113: 5322–5363
Chen B, Wu LZ, Tung CH. Acc Chem Res, 2018, 51: 2512–2523
Song S, Qu J, Han P, Hülsey MJ, Zhang G, Wang Y, Wang S, Chen D, Lu J, Yan N. Nat Commun, 2020, 11: 4899–4908
Nandi J, Leadbeater NE. Org Biomol Chem, 2019, 17: 9182–9186
Romero NA, Nicewicz DA. Chem Rev, 2016, 116: 10075–10166
Liu H, Yan X, Chen W, Xie Z, Li S, Chen W, Zhang T, Xing G, Chen L. Sci China Chem, 2021, 64: 827–833
Huang F, Wang Y, Dong X, Lang X. Sci China Chem, 2023, doi: https://doi.org/10.1007/s11426-023-1644-x
Movassagh B, Shokri S. Tetrahedron Lett, 2005, 46: 6923–6925
Khalafi-Nezhad A, Mohammadi S. RSC Adv, 2014, 4: 13782–13787
Yu L, Li H, Zhang X, Ye J, Liu J, Xu Q, Lautens M. Org Lett, 2014, 16: 1346–1349
Zhang X, Sun J, Ding Y, Yu L. Org Lett, 2015, 17: 5840–5842
Ban YL, Dai JL, Jin XL, Zhang QB, Liu Q. Chem Commun, 2019, 55: 9701–9704
Verma F, Shukla P, Bhardiya SR, Singh M, Rai A, Rai VK. Catal Commun, 2019, 119: 76–81
Zhu C, Ji L, Wei Y. Synthesis, 2010, 18: 3121–3125
Veisi H. Synthesis, 2010, 15: 2631–2635
Wang L, Shen C, Wang H, Zhou W, Sun F, He MY, Chen Q. J Chem Res, 2012, 36: 460–462
Chen GM, Brown HC. J Am Chem Soc, 2000, 122: 4217–4218
Lou S, Moquist PN, Schaus SE. J Am Chem Soc, 2007, 129: 15398–15404
Luo J, Zhang J. ACS Catal, 2016, 6: 873–877
Yang X, Fan Z, Shen Z, Li M. Electrochim Acta, 2017, 226: 53–59
Chen Q, Fang C, Shen Z, Li M. Electrochem Commun, 2016, 64: 51–55
Semmelhack MF, Schmid CR, Cortés DA. Tetrahedron Lett, 1986, 27: 1119–1122
Romero NA, Margrey KA, Tay NE, Nicewicz DA. Science, 2015, 349: 1326–1330
Hu XQ, Chen J, Chen JR, Yan DM, Xiao WJ. Chem Eur J, 2016, 22: 14141–14146
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFA1500100, 2022YFA1502900), the National Natural Science Foundation of China (21933007, 22193013, 22088102), the Strategic Priority Research Program of the Chinese Academy of Science (XDB17000000) and New Cornerstone Science Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information
The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
He, X., Zheng, YW., Chen, B. et al. Metal-free synthesis of nitriles from aldehydes and ammonium by visible-light photocatalysis. Sci. China Chem. 66, 2852–2857 (2023). https://doi.org/10.1007/s11426-023-1748-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1748-4