Abstract
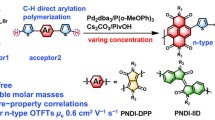
(Hetero)cycloarenes possessing rigid molecular skeletons and large π-systems are the potential active materials in various electronic devices. However, the development of their organic electronics still lags far behind the synthetic chemistry. Herein, in order to bridge this gap, we reported the study of organic semiconductor materials based on heterocycloarenes in detail about the relationship between structure, properties, and device performance. Three varying straight alkyl chain substituted butterfly-shaped heterocycloarenes PTZs were strategically synthesized. Compared with bulky aryl(mesityl) substituted PTZ1, PTZs show additional self-assembly behavior. Concentration-dependent 1H NMR spectra indicated that the self-assembly behavior can be modulated by the alkyl chain length. Medium alkyl chain length substituted heterocycloarene PTZ-C6 showed the strongest association constants of 490 M−1 in solution, and a similar trend was also observed in solid state by thin film absorption spectra. Remarkably, despite the nonplanar conjugated backbones, solution-processing thin film transistor based on PTZ-C6 exhibits hole mobility up to 0.13 cm2 V−1 s−1 and considerable current on/off ratio of 105. Our study demonstrates that substituent engineering of heterocycloarenes is a powerful strategy for modulating self-assembling structures and promoting transistor device performance.

Similar content being viewed by others
References
Diederich F, Staab HA. Angew Chem Int Ed, 1978, 17: 372–374
Buttrick JC, King BT. Chem Soc Rev, 2017, 46: 7–20
Kumar B, Viboh RL, Bonifacio MC, Thompson WB, Buttrick JC, Westlake BC, Kim MS, Zoellner RW, Varganov SA, Mörschel P, Teteruk J, Schmidt MU, King BT. Angew Chem Int Ed, 2012, 51: 12795–12800
Majewski MA, Hong Y, Lis T, Gregoliński J, Chmielewski PJ, Cybińska J, Kim D, Stępień M. Angew Chem Int Ed, 2016, 55: 14072–14076
Fan W, Han Y, Wang X, Hou X, Wu J. J Am Chem Soc, 2021, 143: 13908–13916
Chang D, Zhu J, Sun Y, Chi K, Qiao Y, Wang T, Zhao Y, Liu Y, Lu X. Chem Sci, 2023, 14: 6087–6094
Yang Y, Chu M, Miao Q. Org Lett, 2018, 20: 4259–4262
Zhao M, Pun SH, Gong Q, Miao Q. Angew Chem Int Ed, 2021, 60: 24124–24130
Tatibouët A, Hancock R, Demeunynck M, Lhomme J. Angew Chem Int Ed, 1997, 36: 1190–1191
Yang L, Zhang N, Han Y, Zou Y, Qiao Y, Chang D, Zhao Y, Lu X, Wu J, Liu Y. Chem Commun, 2020, 56: 9990–9993
Zhang N, Yang L, Li W, Zhu J, Chi K, Chang D, Qiao Y, Wang T, Zhao Y, Lu X, Liu Y. J Am Chem Soc, 2022, 144: 21521–21529
Zhang N, Li W, Zhu J, Wang T, Zhang R, Chi K, Liu Y, Zhao Y, Lu X. Adv Mater, 2023, 35: 2300094
Zhu J, Li W, Zhang N, An D, Zhao Y, Lu X, Liu Y. Chem Sci, 2022, 13: 11174–11182
Gregolińska H, Majewski M, Chmielewski PJ, Gregoliński J, Chien A, Zhou J, Wu YL, Bae YJ, Wasielewski MR, Zimmerman PM, Stępień M. J Am Chem Soc, 2018, 140: 14474–14480
Prajapati B, Dang D, Chmielewski PJ, Majewski MA, Lis T, Gómez-García CJ, Zimmerman PM, Stępień M. Angew Chem Int Ed, 2021, 60: 22496–22504
Lu X, An D, Han Y, Zou Y, Qiao Y, Zhang N, Chang D, Wu J, Liu Y. Chem Sci, 2021, 12: 3952–3957
Lu X, Gopalakrishna TY, Phan H, Herng TS, Jiang Q, Liu C, Li G, Ding J, Wu J. Angew Chem Int Ed, 2018, 57: 13052–13056
Lu X, Gopalakrishna TY, Han Y, Ni Y, Zou Y, Wu J. J Am Chem Soc, 2019, 141: 5934–5941
Liu C, Sandoval-Salinas ME, Hong Y, Gopalakrishna TY, Phan H, Aratani N, Herng TS, Ding J, Yamada H, Kim D, Casanova D, Wu J. Chem, 2018, 4: 1586–1595
Zhang L, Colella NS, Liu F, Trahan S, Baral JK, Winter HH, Mannsfeld SCB, Briseno AL. J Am Chem Soc, 2013, 135: 844–854
Osaka I, Zhang R, Sauvé G, Smilgies DM, Kowalewski T, McCullough RD. J Am Chem Soc, 2009, 131: 2521–2529
Izawa T, Miyazaki E, Takimiya K. Adv Mater, 2008, 20: 3388–3392
Cabanetos C, El Labban A, Bartelt JA, Douglas JD, Mateker WR, Fréchet JMJ, McGehee MD, Beaujuge PM. J Am Chem Soc, 2013, 135: 4656–4659
Qiao Y, Yang L, Zhu J, Yan C, Chang D, Zhang N, Zhou G, Zhao Y, Lu X, Liu Y. J Am Chem Soc, 2021, 143: 11088–11101
Back JY, An TK, Cheon YR, Cha H, Jang J, Kim Y, Baek Y, Chung DS, Kwon SK, Park CE, Kim YH. ACS Appl Mater Interfaces, 2015, 7: 351–358
Martin RB. Chem Rev, 1996, 96: 3043–3064
Chen Z, Lohr A, Saha-Möller CR, Würthner F. Chem Soc Rev, 2009, 38: 564–584
Mao L, Hu Y, Tu Q, Jiang WL, Zhao XL, Wang W, Yuan D, Wen J, Shi X. Nat Commun, 2020, 11: 5806
Zhang L, Zeng S, Yin L, Ji C, Li K, Li Y, Wang Y. New J Chem, 2013, 37: 632–639
Liu G, Xiao C, Negri F, Li Y, Wang Z. Angew Chem Int Ed, 2020, 59: 2008–2012
Zhang L, Sun R, Zhang Z, Zhang J, Zhu Q, Ma W, Min J, Wei Z, Deng D. Adv Mater, 2022, 34: 2207020
Vegiraju S, He GY, Kim C, Priyanka P, Chiu YJ, Liu CW, Huang CY, Ni JS, Wu YW, Chen Z, Lee GH, Tung SH, Liu CL, Chen MC, Facchetti A. Adv Funct Mater, 2017, 27: 1606761
Yamaguchi Y, Kojiguchi Y, Kawata S, Mori T, Okamoto K, Tsutsui M, Koganezawa T, Katagiri H, Yasuda T. Chem Mater, 2020, 32: 5350–5360
Becke AD. J Chem Phys, 1993, 98: 5648–5652
Lee C, Yang W, Parr RG. Phys Rev B, 1988, 37: 785–789
Yanai T, Tew DP, Handy NC. Chem Phys Lett, 2004, 393: 51–57
Ditchfield R, Hehre WJ, Pople JA. J Chem Phys, 1971, 54: 724–728
Hehre WJ, Ditchfield R, Pople JA. J Chem Phys, 1972, 56: 2257–2261
Hariharan PC, Pople JA. Theoret Chim Acta, 1973, 28: 213–222
Facchetti A. Mater Today, 2007, 10: 28–37
Wu J, Fechtenkötter A, Gauss J, Watson MD, Kastler M, Fechtenkötter C, Wagner M, Müllen K. J Am Chem Soc, 2004, 126: 11311–11321
Schwab MG, Qin T, Pisula W, Mavrinskiy A, Feng X, Baumgarten M, Kim H, Laquai F, Schuh S, Trattnig R W. List EJ, Müllen K. Chem Asian J, 2011, 6: 3001–3010
He Z, Xu X, Zheng X, Ming T, Miao Q. Chem Sci, 2013, 4: 4525–4531
Bayer J, Huhn T. J Org Chem, 2022, 87: 5257–5278
Gu PY, Zhang J, Long G, Wang Z, Zhang Q. J Mater Chem C, 2016, 4: 3809–3814
Yi Z, Sun X, Zhao Y, Guo Y, Chen X, Qin J, Yu G, Liu Y. Chem Mater, 2012, 24: 4350–4356
Liu Q, Kumagai S, Manzhos S, Chen Y, Angunawela I, Nahid MM, Feron K, Bottle SE, Bell J, Ade H, Takeya J, Sonar P. Adv Funct Mater, 2020, 30: 2000489
Sun B, Hong W, Yan Z, Aziz H, Li Y. Adv Mater, 2014, 26: 2636–2642
Chen CA, Yang PC, Wang SC, Tung SH, Su WF. Macromolecules, 2018, 51: 7828–7835
Khatun MN, Dey A, Meher N, Iyer PK. ACS Appl Electron Mater, 2021, 3: 3575–3587
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52073063, 61890940), the National Key R&D Program of China (2018YFA0703200), the Natural Science Foundation of Shanghai (22ZR1405800 and 23ZR1405100), the Program for Professor of Special Appointment (Eastern Scholar) at the Shanghai Institutions of Higher Learning.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at https://chem.scichina.com and https://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Zhu, J., Li, W., Zhang, R. et al. Effect of substituents on self-assembling behaviors and charge transport properties of nonplanar heterocycloarenes. Sci. China Chem. 66, 2903–2911 (2023). https://doi.org/10.1007/s11426-023-1743-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1743-4