Abstract
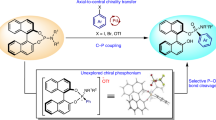
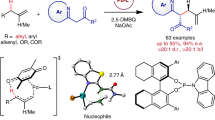
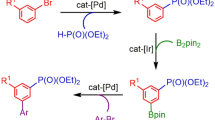
Chiral phosphine-containing skeletons play a pivotal role in bioactive natural products, pharmaceuticals, chiral catalysts, and ligands. Despite considerable progress has been made in the synthesis of chiral phosphorus compounds, the development of facile and modular methods to access chiral allylic phosphorus compounds remains challenging due to the simultaneous control required for reactivity, enantioselectivity, and stereoselectivity. Herein, we present a general and modular platform to achieve the asymmetric reductive cross-coupling of α-bromophosphonates and vinyl bromides, enabling the synthesis of highly valuable chiral allylic phosphonate products with remarkable yields, enantioselectivities, and stereoselectivities.

Similar content being viewed by others
Change history
19 October 2023
The corresponding author Tao Xu to Tao XU has been updated.
References
Zhou QL. Privileged Chiral Ligands and Catalysts. Weinheim: Wiley-VCH, 2011. 55–91
Hu HL, Ren X, He J, Zhu L, Fang S, Su Z, Wang T. Sci China Chem, 2022, 65: 2500–2511
Belal M, Li Z, Lu X, Yin G. Sci China Chem, 2021, 64: 513–533
Hecker SJ, Erion MD. J Med Chem, 2008, 51: 2328–2345
Sheng XC, Pyun HJ, Chaudhary K, Wang J, Doerffler E, Fleury M, McMurtrie D, Chen X, Delaney Iv WE, Kim CU. Bioorg Med Chem Lett, 2009, 19: 3453–3457
Wiemer AJ. ACS Pharmacol Transl Sci, 2020, 3: 613–626
Jia X, Schols D, Meier C. J Med Chem, 2020, 63: 6003–6027
Butti P, Rochat R, Sadow A, Togni A. Angew Chem Int Ed, 2008, 47: 4878–4881
Zhang L, Liu W, Zhao X. Eur J Org Chem, 2014, 2014(31): 6846–6849
Nie SZ, Davison RT, Dong VM. J Am Chem Soc, 2018, 140: 16450–16454
Long J, Li Y, Zhao W, Yin G. Chem Sci, 2022, 13: 1390–1397
Yang Z, Wang JJ. Angew Chem Int Ed, 2021, 60: 27288–27292
Li B, Liu M, Rehman SU, Li C. J Am Chem Soc, 2022, 144: 2893–2898
Nishimura T, Hirabayashi S, Yasuhara Y, Hayashi T. J Am Chem Soc, 2006, 128: 2556–2557
Nishimura T, Guo XX, Hayashi T. Chem Asian J, 2008, 3: 1505–1510
Yang XY, Tay WS, Li Y, Pullarkat SA, Leung PH. Organometallics, 2015, 34: 5196–5201
Hong L, Sun W, Liu C, Zhao D, Wang R. Chem Commun, 2010, 46: 2856–2858
Sun W, Hong L, Liu C, Wang R. Org Lett, 2010, 12: 3914–3917
Hatano M, Horibe T, Ishihara K. Angew Chem Int Ed, 2013, 52: 4549–4553
Choi J, Fu GC. J Am Chem Soc, 2012, 134: 9102–9105
Choi J, Martín-Gago P, Fu GC. J Am Chem Soc, 2014, 136: 12161–12165
Lou S, Fu GC. J Am Chem Soc, 2010, 132: 5010–5011
Cherney AH, Reisman SE. J Am Chem Soc, 2014, 136: 14365–14368
Suzuki N, Hofstra JL, Poremba KE, Reisman SE. Org Lett, 2017, 19: 2150–2153
Hofstra JL, Cherney AH, Ordner CM, Reisman SE. J Am Chem Soc, 2018, 140: 139–142
DeLano TJ, Reisman SE. ACS Catal, 2019, 9: 6751–6754
Wu L, Wei H, Shen J, Chen J, Zhang W. Acta Chim Sin, 2021, 79: 1331–1344
Cheng X, Li T, Liu Y, Lu Z. ACS Catal, 2021, 11: 11059–11065
Xu J, Li Z, Xu Y, Shu X, Huo H. ACS Catal, 2021, 11: 13567–13574
Liu J, Gong H, Zhu S. Angew Chem Int Ed, 2021, 60: 4060–4064
Jin RX, Wu BB, Bian KJ, Yu JL, Dai JC, Zuo YW, Zhang YF, Wang XS. Nat Commun, 2022, 13: 7035
He SJ, Wang JW, Li Y, Xu ZY, Wang XX, Lu X, Fu Y. J Am Chem Soc, 2020, 142: 214–221
Li X, Yuan M, Chen F, Huang Z, Qing FL, Gutierrez O, Chu L. Chem, 2023, 9: 154–169
Wang H, Zheng P, Wu X, Li Y, Xu T. J Am Chem Soc, 2022, 144: 3989–3997
Wang H, Wu X, Xu T. Angew Chem Int Ed, 2023, 62: e202218299
Zhou J, Wang D, Xu W, Hu Z, Xu T. J Am Chem Soc, 2023, 145: 2081–2087
Lin D, Chen Y, Dong Z, Pei P, Ji H, Tai L, Chen LA. CCS Chem, 2023, 5: 1386–1397
Lin Q, Dawson G, Diao T. Synlett, 2021, 32: 1606–1620
Turro RF, Wahlman JLH, Tong ZJ, Chen X, Yang M, Chen EP, Hong X, Hadt RG, Houk KN, Yang YF, Reisman SE. JAm Chem Soc, 2023, 145: 14705–14715
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071183) and the Science and Technology Commission of Shanghai Municipality (19DZ2271500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at https://chem.scichina.com and https://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information for
11426_2023_1726_MOESM1_ESM.pdf
Synthesis of Chiral Allylic Phosphonates via Asymmetric Reductive Cross-Coupling of α-Bromophosphonates and Vinyl Bromides
Rights and permissions
About this article
Cite this article
Wang, H., Li, X. & XU, T. Synthesis of chiral allylic phosphonates via asymmetric reductive cross-coupling of α-bromophosphonates and vinyl bromides. Sci. China Chem. 66, 2621–2625 (2023). https://doi.org/10.1007/s11426-023-1726-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1726-1