Abstract
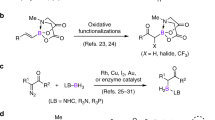
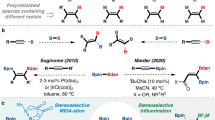
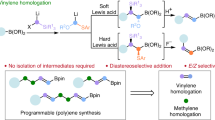
α-Functionalized organoborons are useful building blocks and key structural elements in functional molecules. Their previous synthesis relied on the famous Matteson reaction or the late-stage borylative modification of alkynes or alkenes. Recently, the synthetic transformation of borylated building blocks offers another useful strategy and is currently actively explored. We report herein that B(MIDA)-propargylic alcohols (BPAs) are a useful type of borylated building blocks. Bearing two complementary functional group handles (alkyne and hydroxyl) in close proximity, the redox-neutral [3,3] and [2,3] sigmatropic rearrangements of BPAs allow the efficient synthesis of several types of α-functionalized boronates, including α,β-unsaturated acylborons, α-S/P-substituted allenylborons, boryl-substituted thiazoles and a borylated α,β-unsaturated hydrazine, some of which are otherwise challenging targets using other synthetic methods.

Similar content being viewed by others
References
Hall DG. Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials. 2nd ed. Weinheim: Wiley-VCH, 2011
Andrés P, Ballano G, Calaza MI, Cativiela C. Chem Soc Rev, 2016, 45: 2291–2307
Matteson DS. J Org Chem, 2013, 78: 10009–10023
Xie Q, Dong G. J Am Chem Soc, 2021, 143: 14422–14427
Jin JK, Zheng WX, Xia HM, Zhang FL, Wang YF. Org Lett, 2019, 21: 8414–8418
Feng Q, Li S, Li Z, Yan Q, Lin X, Song L, Zhang X, Wu YD, Sun J. J Am Chem Soc, 2022, 144: 14846–14855
Ma X, Kuang Z, Song Q. JACS Au, 2022, 2: 261–279
Volochnyuk DM, Gorlova AO, Grygorenko OO. Chem Eur J, 2021, 27: 15277–15326
Aich D, Kumar P, Ghorai D, Kanti Das K, Panda S. Chem Commun, 2022, 58: 13298–13316
Gillis EP, Burke MD. J Am Chem Soc, 2007, 129: 6716–6717
Mancilla T, Contreras R, Wrackmeyer B. J Organomet Chem, 1986, 307: 1–6
He Z, Yudin AK. J Am Chem Soc, 2011, 133: 13770–13773
Li J, Burke MD. J Am Chem Soc, 2011, 133: 13774–13777
Diaz DB, Scully CCG, Liew SK, Adachi S, Trinchera P, St. Denis JD, Yudin AK. Angew Chem Int Ed, 2016, 55: 12659–12663
Ivon YM, Mazurenko IV, Kuchkovska YO, Voitenko ZV, Grygorenko OO. Angew Chem Int Ed, 2020, 59: 18016–18022
Soor HS, Diaz DB, Burton KI, Yudin AK. Angew Chem Int Ed, 2021, 60: 16366–16371
Lee CF, Holownia A, Bennett JM, Elkins JM, St. Denis JD, Adachi S, Yudin AK. Angew Chem Int Ed, 2017, 56: 6264–6267
Lv WX, Zeng YF, Li Q, Chen Y, Tan DH, Yang L, Wang H. Angew Chem Int Ed, 2016, 55: 10069–10073
Molander GA, Raushel J, Ellis NM. J Org Chem, 2010, 75: 4304–4306
Scharnagl FK, Bose SK, Marder TB. Org Biomol Chem, 2017, 15: 1738–1752
Osuna Gálvez A, Bode JW. J Am Chem Soc, 2019, 141: 8721–8726
Taguchi J, Takeuchi T, Takahashi R, Masero F, Ito H. Angew Chem Int Ed, 2019, 58: 7299–7303
Tien CH, Trofimova A, Holownia A, Kwak BS, Larson RT, Yudin AK. Angew Chem Int Ed, 2021, 60: 4342–4349
Zeng YF, Ji WW, Lv WX, Chen Y, Tan DH, Li Q, Wang H. Angew Chem Int Ed, 2017, 56: 14707–14711
Lv WX, Li Q, Li JL, Li Z, Lin E, Tan DH, Cai YH, Fan WX, Wang H. Angew Chem Int Ed, 2018, 57: 16544–16548
Wang Q, Biosca M, Himo F, Szabó KJ. Angew Chem Int Ed, 2021, 60: 26327–26331
Liu Y, Chen ZH, Li Y, Qian J, Li Q, Wang H. JAm Chem Soc, 2022, 144: 14380–14387
Lee SJ, Anderson TM, Burke MD. Angew Chem Int Ed, 2010, 49: 8860–8863
Tan DH, Cai YH, Zeng YF, Lv WX, Yang L, Li Q, Wang H. Angew Chem Int Ed, 2019, 58: 13784–13788
Gálvez AO, Schaack CP, Noda H, Bode JW. J Am Chem Soc, 2017, 139: 1826–1829
Zhu Y, Sun L, Lu P, Wang Y. ACS Catal, 2014, 4: 1911–1925
Qian J, Chen ZH, Liu Y, Li Y, Li Q, Huang SL, Wang H. Chin Chem Lett, 2023, 34: 107479
He Z, Trinchera P, Adachi S, St. Denis JD, Yudin AK. Angew Chem Int Ed, 2012, 51: 11092–11096
Lepage ML, Lai S, Peressin N, Hadjerci R, Patrick BO, Perrin DM. Angew Chem Int Ed, 2017, 56: 15257–15261
Wu D, Fohn NA, Bode JW. Angew Chem Int Ed, 2019, 58: 11058–11062
Cheng LJ, Zhao S, Mankad NP. Angew Chem IntEd, 2021, 60: 2094–2098
Lin S, Wang L, Sharma A. Chem Sci, 2021, 12: 7924–7929
Schuhmacher A, Ryan SJ, Bode JW. Angew Chem Int Ed, 2021, 60: 3918–3922
Tung P, Schuhmacher A, Schilling PE, Bode JW, Mankad NP. Angew Chem Int Ed, 2022, 61: e202114513
Ibrahem I, Breistein P, Cordova A. Chem Eur J, 2012, 18: 5175–5179
Cadierno V, Crochet P, Garcia-Garrido SE, Gimeno J. Dalton Trans, 2010, 39: 4015–4031
Nikolaev A, Orellana A. Org Lett, 2015, 17: 5796–5799
Bellemin-Laponnaz S, Gisie H, Le Ny JP, Osborn JA. Angew Chem Int Ed, 1997, 36: 976–978
Meinhardt NA, Boisselle AP. J Org Chem, 1961, 27: 1828–1833
Torres LC, Dobrovetsky R, Caputo CB. Chem Commun, 2021, 57: 8272–8275
Takeshi N, Akira A-I. Tetrahedron Lett, 1976, 27: 2335–2338
Harusawa S, Moriyama H, Kase N, Ohishi H, Yoneda R, Kurihara T. Tetrahedron, 1995, 51: 6475–6494
Huang MY, Zhao YT, Zhang CD, Zhu SF. Angew Chem Int Ed, 2022, 61: e202203343
Gao X, Pan Y, Lin M, Chen L, Zhan Z. Org Biomol Chem, 2010, 8: 3259–3266
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22022114, 21971261), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Y093), and Guangdong Basic Research Center of Excellence for Functional Molecular Engineering. This work is dedicated to the 20th Anniversary of School of Pharmaceutical Sciences, Sun Yat-sen University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at chem.scichina.com and link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2023_1722_MOESM1_ESM.pdf
Sigmatropic Rearrangements of B(MIDA)-Propargylic Alcohols towards the Diverse Synthesis of α-Functionalized Organoborons
Rights and permissions
About this article
Cite this article
Qian, J., Liu, LC., Chen, ZH. et al. Sigmatropic rearrangements of B(MIDA)-propargylic alcohols towards the diverse synthesis of α-functionalized organoborons. Sci. China Chem. 67, 568–575 (2024). https://doi.org/10.1007/s11426-023-1722-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1722-8