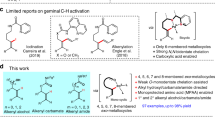
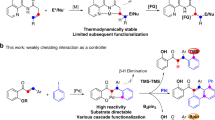
Abstract
Catalyst innovation lies at the heart of transition-metal-catalyzed reaction development. In this article, we have explored the C(sp2)—H alkenylation activity with novel spirocyclic N-heterocyclic carbene (NHC)-based cyclometalated ruthenium pincer catalyst system, SNRu-X. After screening catalyst and condition, a high valent Ru(IV) dioxide (X = O2) species has demonstrated superior reactivity in the catalytic alkenylation of aromatic and olefinic C-H bonds with unactivated alkenyl bromides and triflates. This reaction has achieved the easy construction of a wide range of (hetero)aromatic alkenes and dienes, in good to excellent yields with exclusive selectivity. Preliminary mechanistic studies indicate that this reaction may proceed through a single electron transfer (SET) triggered oxidative addition, by doing so, providing valuable complementary to classical alke-nylation reactions that are dependent on activated alkenyl precursors.

Similar content being viewed by others
References
Elsby MR, Baker RT. Chem Soc Rev, 2020, 49: 8933–8987
Ibáñez S, Poyatos M, Peris E. Acc Chem Res, 2020, 53: 1401–1413
Rogge T, Kaplaneris N, Chatani N, Kim J, Chang S, Punji B, Schafer LL, Musaev DG, Wencel-Delord J, Roberts CA, Sarpong R, Wilson ZE, Brimble MA, Johansson MJ, Ackermann L. Nat Rev Methods Primers, 2021, 1: 43
Ott JC, Bürgy D, Guan H, Gade LH. Acc Chem Res, 2022, 55: 857–868
Marciniec B, Pietraszuk C, Pawluć P, Maciejewski H. Chem Rev, 2022, 122: 3996–4090
Hartwig JF. Acc Chem Res, 2012, 45: 864–873
Guisado-Barrios G, Soleilhavoup M, Bertrand G. Acc Chem Res, 2018, 51: 3236–3244
Soleilhavoup M, Bertrand G. Chem, 2020, 6: 1275–1282
Jazzar R, Soleilhavoup M, Bertrand G. Chem Rev, 2020, 120: 4141–4168
Yazdani S, Junor GP, Peltier JL, Gembicky M, Jazzar R, Grotjahn DB, Bertrand G. ACS Catal, 2020, 10: 5190–5201
Gao Y, Kim N, Mendoza SD, Yazdani S, Faria Vieira A, Liu M, Kendrick IV A, Grotjahn DB, Bertrand G, Jazzar R, Engle KM. ACS Catal, 2022, 12: 7243–7247
Wang Y, Huang Z, Liu G, Huang Z. Acc Chem Res, 2022, 55: 2148–2161
Yang BM, Xiang K, Tu YQ, Zhang SH, Yang DT, Wang SH, Zhang FM. Chem Commun, 2014, 50: 7163–7165
Yan ZB, Peng M, Chen QL, Lu K, Tu YQ, Dai KL, Zhang FM, Zhang XM. Chem Sci, 2021, 12: 9748–9753
Dai K, Chen Q, Xie W, Lu K, Yan Z, Peng M, Li C, Tu Y, Ding T. Angew Chem Int Ed, 2022, 61: e202206446
Palladium, Iridium, Rhodium and Ruthenium, Monthly Average Prices between 1st December 2017 and 1st December 2022. Johnson Matthey Precious Metals Management Home Page. http://www.platinum.matthey.com/prices/price-charts (accessed on 2022-12-15)
Arockiam PB, Bruneau C, Dixneuf PH. Chem Rev, 2012, 112: 5879–5918
Leitch JA, Frost CG. Chem Soc Rev, 2017, 46: 7145–7153
Nareddy P, Jordan F, Szostak M. ACS Catal, 2017, 7: 5721–5745
Korvorapun K, Samanta RC, Rogge T, Ackermann L. Synthesis, 2021, 53: 2911–2946
Findlay MT, Domingo-Legarda P, McArthur G, Yen A, Larrosa I. Chem Sci, 2022, 13: 3335–3362
Ackermann L. Org Lett, 2005, 7: 3123–3125
Ackermann L, Born R, Álvarez-Bercedo P. Angew Chem Int Ed, 2007, 46: 6364–6367
Ackermann L, Vicente R, Potukuchi HK, Pirovano V. Org Lett, 2010, 12: 5032–5035
Kakiuchi F, Kochi T, Mizushima E, Murai S. J Am Chem Soc, 2010, 132: 17741–17750
Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Köhn G, Whittlesey MK, Frost CG. J Am Chem Soc, 2011, 133: 19298–19301
Hofmann N, Ackermann L. J Am Chem Soc, 2013, 135: 5877–5884
Juliá-Hernández F, Simonetti M, Larrosa I. Angew Chem Int Ed, 2013, 52: 11458–11460
Simonetti M, Perry GJP, Cambeiro XC, Juliá-Hernández F, Arokianathar JN, Larrosa I. J Am Chem Soc, 2016, 138: 3596–3606
Simonetti M, Cannas DM, Just-Baringo X, Vitorica-Yrezabal IJ, Larrosa I. Nat Chem, 2018, 10: 724–731
Simonetti M, Kuniyil R, Macgregor SA, Larrosa I. J Am Chem Soc, 2018, 140: 11836–11847
Wang GW, Wheatley M, Simonetti M, Cannas DM, Larrosa I. Chem, 2020, 6: 1459–1468
Korvorapun K, Kuniyil R, Ackermann L. ACS Catal, 2020, 10: 435–440
Korvorapun K, Struwe J, Kuniyil R, Zangarelli A, Casnati A, Waeterschoot M, Ackermann L. Angew Chem Int Ed, 2020, 59: 18103–18109
Spencer ARA, Korde R, Font M, Larrosa I. Chem Sci, 2020, 11: 4204–4208
Sagadevan A, Charitou A, Wang F, Ivanova M, Vuagnat M, Greaney MF. Chem Sci, 2020, 11: 4439–4443
Wheatley M, Findlay MT, López-Rodríguez R, Cannas DM, Simonetti M, Larrosa I. Chem Catal, 2021, 1: 691–703
Liu KKC, Li J, Sakya S. MRMC, 2004, 4: 1105–1125
Negishi E, Huang Z, Wang G, Mohan S, Wang C, Hattori H. Acc Chem Res, 2008, 41: 1474–1485
Negishi E. Angew Chem Int Ed, 2011, 50: 6738–6764
Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ. Chem Rev, 2015, 115: 11718–11940
Schipper DJ, Hutchinson M, Fagnou K. J Am Chem Soc, 2010, 132: 6910–6911
Gao K, Lee PS, Fujita T, Yoshikai N. J Am Chem Soc, 2010, 132: 12249–12251
Zhou B, Chen H, Wang C. J Am Chem Soc, 2013, 135: 1264–1267
Halbritter G, Knoch F, Wolski A, Kisch H. Angew Chem Int Ed Engl, 1994, 33: 1603–1605
Arockiam PB, Fischmeister C, Bruneau C, Dixneuf PH. Green Chem, 2011, 13: 3075–3078
Wang C, Chen H, Wang Z, Chen J, Huang Y. Angew Chem Int Ed, 2012, 51: 7242–7245
Zhang H, Yang Z, Liu J, Yu X, Wang Q, Wu Y. Org Chem Front, 2019, 6: 967–971
Zhang J, Lu X, Shen C, Xu L, Ding L, Zhong G. Chem Soc Rev, 2021, 50: 3263–3314
Matsuura Y, Tamura M, Kochi T, Sato M, Chatani N, Kakiuchi F. J Am Chem Soc, 2007, 129: 9858–9859
Ye W, Luo N, Yu Z. Organometallics, 2010, 29: 1049–1052
Ogiwara Y, Tamura M, Kochi T, Matsuura Y, Chatani N, Kakiuchi F. Organometallics, 2014, 33: 402–420
Otley KD, Ellman JA. Org Lett, 2015, 17: 1332–1335
Prakash S, Muralirajan K, Cheng CH. Chem Commun, 2015, 51: 13362–13364
Zhao H, Xu X, Luo Z, Cao L, Li B, Li H, Xu L, Fan Q, Walsh PJ. Chem Sci, 2019, 10: 10089–10096
Jiang X, Zeng Z, Hua Y, Xu B, Shen Y, Xiong J, Qiu H, Wu Y, Hu T, Zhang Y. J Am Chem Soc, 2020, 142: 15585–15594
Herein, we refer styrene halides (possessing a conjugated (system) and acrylates halides (possessing an electron withdrawing activating group) as the activated alkenyl halides, and alkenyl halides without these activating factors, for example alkyl substituted electron neutral alkenyl halides, as unactivated alkenyl halides. Indeed, they showed significant lower reactivity in C-H bond alkenylation, see: (a) Gottumukkala AL, Derridj F, Djebbar S, Doucet H. Tetrahedron Lett, 2008, 49: 2926–2930
Schneider C, Masi D, Couve-Bonnaire S, Pannecoucke X, Hoarau C. Angew Chem Int Ed, 2013, 52: 3246–3249
Zhang W, Tian Y, Zhao N, Wang Y, Li J, Wang Z. Tetrahedron, 2014, 70: 6120–6126
Zhao Q, Tognetti V, Joubert L, Besset T, Pannecoucke X, Bouillon JP, Poisson T. Org Lett, 2017, 19: 2106–2109
Casotti G, Fusini G, Ferreri M, Pardini LF, Evangelisti C, Angelici G, Carpita A. Synthesis, 2020, 52: 1795–1803
Feng G, Liu B. Acc Chem Res, 2018, 51: 1404–1414
Suleymanov AA, Doll M, Ruggi A, Scopelliti R, Fadaei-Tirani F, Severin K. Angew Chem Int Ed, 2020, 59: 9957–9961
(a)_Azpíroz R, Di Giuseppe A, Passarelli V, Pérez-Torrente JJ, Oro LA, Castarlenas R. Organometallics, 2018, 37: 1695–1707
Yan R, Wang ZX. Asian J Org Chem, 2018, 7: 240–247
Pagire SK, Föll T, Reiser O. Acc Chem Res, 2020, 53: 782–791
Simmons EM, Hartwig JF. Angew Chem Int Ed, 2012, 51: 3066–3072
According to Tolman’s calculation of electronic parameter, the nucleophility of our spirocyclic 6-membered ring’s NHC ligand is much higher than the commomly used 5-membered ring’s imidazole-type NHCs, ranking the second of two top NHCs species so far reported. See: Tolman CA. Chem Rev, 1977, 77: 313–348
Acknowledgements
This work was suported by the National Natural Science Foundation of China (2187,1117, 91956203), the “111” Program of Minister of Education, Beijing National Laboratory for Molecular Sciences (BNLMS202109) and the Science and Technology Commission of Shanghai Municipality (19JC1430100). We also thank Prof. Lu Zhan (Zhejiang University) for his kind assistant HRMS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
Rights and permissions
About this article
Cite this article
Cai, H., Tu, YQ., Lu, K. et al. Aromatic and olefinic C-H alkenylation by catalysis with spirocyclic NHC Ru(IV) pincer complex. Sci. China Chem. 66, 2791–2796 (2023). https://doi.org/10.1007/s11426-022-1541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1541-5