Abstract
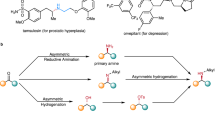
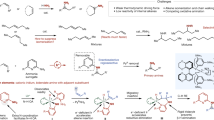
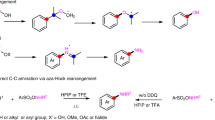
Direct reductive amination (DRA) is one of the most efficient methods for amine synthesis. Herein we report a practical homogeneous DRA procedure utilizing iridium catalysis. Applying simple, readily available and inexpensive PPh3 and alike ligands along with iridium at a low loading, aldehydes and ketones reductively coupled with primary and secondary amines to efficiently form structurally and functionally diverse amine products, including a set of drugs and compounds from late-stage manipulation. The reaction conditions were exceptionally mild and additive-free, in which oxygen, moisture, polar protic groups and multiple other functional groups were tolerated. For targeted products, this methodology is especially versatile for offering multiple possible synthetic options. The 10 gram-scale synthesis further demonstrated the potential and promise of this procedure in practical amine synthesis. DFT studies reveal an “outer-sphere” H-addition pathway, in which π−π interactions and H-bonding play important roles.

Similar content being viewed by others
References
Ricci A. Modern Amination Methods. Weinheim: Wiley-VCH, 2000
Lawrence SA. Amines: Synthesis, Properties and Applications. Cambridge: Cambridge University Press, 2004
Ricci A. Amino Group Chemistry: From Synthesis to the Life Sciences. Weinheim: Wiley-VCH, 2008
Froidevaux V, Negrell C, Caillol S, Pascault JP, Boutevin B. Chem Rev, 2016, 116: 14181–14224
Vitaku E, Smith DT, Njardarson JT. J Med Chem, 2014, 57: 10257–10274
Roughley SD, Jordan AM. J Med Chem, 2011, 54: 3451–3479
Blakemore DC, Castro L, Churcher I, Rees DC, Thomas AW, Wilson DM, Wood A. Nat Chem, 2018, 10: 383–394
Brown DG, Boström J. J Med Chem, 2016, 59: 4443–4458
Trowbridge A, Walton SM, Gaunt MJ. Chem Rev, 2020, 120: 2613–2692
Leuckart R. Ber Dtsch Chem Ges, 1885, 18: 2341–2344
Podyacheva E, Afanasyev OI, Tsygankov AA, Makarova M, Chusov D. Synthesis, 2019, 51: 2667–2677
Afanasyev OI, Kuchuk E, Usanov DL, Chusov D. Chem Rev, 2019, 119: 11857–11911
Irrgang T, Kempe R. Chem Rev, 2020, 120: 9583–9674
Murugesan K, Senthamarai T, Chandrashekhar VG, Natte K, Kamer PCJ, Beller M, Jagadeesh RV. Chem Soc Rev, 2020, 49: 6273–6328
Tian Y, Hu L, Wang YZ, Zhang X, Yin Q. Org Chem Front, 2021, 8: 2328–2342
Reshi NUD, Saptal VB, Beller M, Bera JK. ACS Catal, 2021, 11: 13809–13837
Jagadeesh RV, Murugesan K, Alshammari AS, Neumann H, Pohl MM, Radnik J, Beller M. Science, 2017, 358: 326–332
Murugesan K, Beller M, Jagadeesh RV. Angew Chem Int Ed, 2019, 58: 5064–5068
Li C, Villa-Marcos B, Xiao J. J Am Chem Soc, 2009, 131: 6967–6969
Steinhuebel D, Sun Y, Matsumura K, Sayo N, Saito T. J Am Chem Soc, 2009, 131: 11316–11317
Chen ZP, Hu SB, Zhou J, Zhou YG. ACS Catal, 2015, 5: 6086–6089
Huang H, Liu X, Zhou L, Chang M, Zhang X. Angew Chem Int Ed, 2016, 55: 5309–5312
Yang P, Lim LH, Chuanprasit P, Hirao H, Zhou JS. Angew Chem Int Ed, 2016, 55: 12083–12087
Lou Y, Hu Y, Lu J, Guan F, Gong G, Yin Q, Zhang X. Angew Chem Int Ed, 2018, 57: 14193–14197
Tan X, Gao S, Zeng W, Xin S, Yin Q, Zhang X. J Am Chem Soc, 2018, 140: 2024–2027
Gallardo-Donaire J, Hermsen M, Wysocki J, Ernst M, Rominger F, Trapp O, Hashmi ASK, Schäfer A, Comba P, Schaub T. J Am Chem Soc, 2018, 140: 355–361
Wu Z, Du S, Gao G, Yang W, Yang X, Huang H, Chang M. Chem Sci, 2019, 10: 4509–4514
Hu L, Zhang Y, Zhang Q, Yin Q, Zhang X. Angew Chem Int Ed, 2020, 59: 5321–5325
Yuan S, Gao G, Wang L, Liu C, Wan L, Huang H, Geng H, Chang M. Nat Commun, 2020, 11: 621
Gao Z, Liu J, Huang H, Geng H, Chang M. Angew Chem Int Ed, 2021, 60: 27307–27311
Wu Z, Wang W, Guo H, Gao G, Huang H, Chang M. Nat Commun, 2022, 13: 3344
Dorkó É, Szabó M, Kótai B, Pápai I, Domján A, Soós T. Angew Chem Int Ed, 2017, 56: 9512–9516
Senthamarai T, Murugesan K, Schneidewind J, Kalevaru NV, Baumann W, Neumann H, Kamer PCJ, Beller M, Jagadeesh RV. Nat Commun, 2018, 9: 4123–4134
Murugesan K, Wei Z, Chandrashekhar VG, Neumann H, Spannenberg A, Jiao H, Beller M, Jagadeesh RV. Nat Commun, 2019, 10: 5443
Wang C, Pettman A, Basca J, Xiao J. Angew Chem Int Ed, 2010, 49: 7548–7552
Tanaka K, Miki T, Murata K, Yamaguchi A, Kayaki Y, Kuwata S, Ikariya T, Watanabe M. J Org Chem, 2019, 84: 10962–10977
Polishchuk I, Sklyaruk J, Lebedev Y, Rueping M. Chem Eur J, 2021, 27: 5919–5922
Sagan S, Karoyan P, Lequin O, Chassaing G, Lavielle S. Curr Med Chem, 2004, 11: 2799–2822
Subtelny AO, Hartman MCT, Szostak JW. J Am Chem Soc, 2008, 130: 6131–6136
Lee OY, Law KL, Ho CY, Yang D. J Org Chem, 2008, 73: 8829–8837
Nayal OS, Bhatt V, Sharma S, Kumar N. J Org Chem, 2015, 80: 5912–5918
Varjosaari SE, Skrypai V, Suating P, Hurley JJM, Lio AMD, Gilbert TM, Adler MJ. Adv Synth Catal, 2017, 359: 1872–1878
Isidro-Llobet A, Álvarez M, Albericio F. Chem Rev, 2009, 109: 2455–2504
Aurelio L, Brownlee RTC, Hughes AB. Chem Rev, 2004, 104: 5823–5846
Dobereiner GE, Nova A, Schley ND, Hazari N, Miller SJ, Eisenstein O, Crabtree RH. J Am Chem Soc, 2011, 133: 7547–7562
Li ML, Yang S, Su XC, Wu HL, Yang LL, Zhu SF, Zhou QL. J Am Chem Soc, 2017, 139: 541–547
Cui CX, Chen H, Li SJ, Zhang T, Qu LB, Lan Y. Coord Chem Rev, 2020, 412: 213251
Grimme S. Angew Chem Int Ed, 2008, 47: 3430–3434
Martinez CR, Iverson BL. Chem Sci, 2012, 3: 2191–2201
Carter-Fenk K, Herbert JM. Phys Chem Chem Phys, 2020, 22: 24870–24886
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21772155), the National Key R&D Program of China (2020YFA0907901), the Scientific Fund of Northwest A&F University and Postdoctoral Science Foundation of China (2019M663827). We thank HPC of Northwest A&F University for the DFT calculations carried out in this work. We also give special thanks to Dr. Xiuhuan Li of State Key Laboratory of Crop Stress Biology for Arid Areas at Northwest A&F University for her kind help on the NMR spectroscopy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at https://chem.scichina.com and https://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information for
Rights and permissions
About this article
Cite this article
Wang, J., Wang, W., Yang, X. et al. Practical N-alkylation via homogeneous iridium-catalyzed direct reductive amination. Sci. China Chem. 66, 518–525 (2023). https://doi.org/10.1007/s11426-022-1494-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1494-7