Abstract
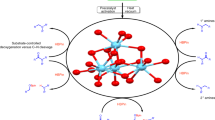
The reduction of carboxamides into high value-added amines is a very interesting but great challenging topic. Herein we demonstrate that polynuclear lanthanide-oxo clusters Ln16 (Ln = Eu and Gd) can be used as efficient catalyst to reduce primary and secondary carboxamides to amines with excellent yield of 71%–98% and broad substrates scope. The methodology can extend to the gram-scale synthesis of phenethylamine drug with 93% yield. Based on the isolation and characterization of catalytic intermediates, a catalytic mechanism involving multipath reaction is proposed. This work provides efficient lanthanide cluster catalysts for the reduction of carboxamides to amines.

Similar content being viewed by others
References
Kuwano R, Takahashi M, Ito Y. Tetrahedron Lett, 1998, 39: 1017–1020
Tamang SR, Singh A, Bedi D, Bazkiaei AR, Warner AA, Glogau K, McDonald C, Unruh DK, Findlater M. Nat Catal, 2020, 3: 154–162
Bhunia M, Sahoo SR, Das A, Ahmed J, P. S, Mandal SK. Chem Sci, 2020, 11: 1848–1854
Balaraman E, Gnanaprakasam B, Shimon LJW, Milstein D. J Am Chem Soc, 2010, 132: 16756–16758
Chardon A, Morisset E, Rouden J, Blanchet J. Synthesis, 2018, 50: 984–997
Volkov A, Tinnis F, Slagbrand T, Trillo P, Adolfsson H. Chem Soc Rev, 2016, 45: 6685–6697
Saha S, Eisen MS. Dalton Trans, 2020, 49: 12835–12841
Liu RY, Bae M, Buchwald SL. J Am Chem Soc, 2018, 140: 1627–1631
Kuciński K, Hreczycho G. Green Chem, 2020, 22: 5210–5224
Huang PQ, Lang QW, Wang YR. J Org Chem, 2016, 81: 4235–4243
Huang PQ, Geng H. Org Chem Front, 2015, 2: 150–158
Papa V, Cabrero-Antonino JR, Alberico E, Spanneberg A, Junge K, Junge H, Beller M. Chem Sci, 2017, 8: 3576–3585
Sitte NA, Bursch M, Grimme S, Paradies J. J Am Chem Soc, 2019, 141: 159–162
Chaudhari MB, Gnanaprakasam B. Chem Asian J, 2019, 14: 76–93
Yao W, Wang J, Zhong A, Wang S, Shao Y. Org Chem Front, 2020, 7: 3515–3520
Das HS, Das S, Dey K, Singh B, K. Haridasan R, Das A, Ahmed J, Mandal SK. Chem Commun, 2019, 55: 11868–11871
Yu C, Guo C, Jiang L, Gong M, Luo Y. Organometallics, 2021, 40: 1201–1206
Sunada Y, Kawakami H, Imaoka T, Motoyama Y, Nagashima H. Angew Chem Int Ed, 2009, 48: 9511–9514
Das S, Addis D, Zhou S, Junge K, Beller M. J Am Chem Soc, 2010, 132: 1770–1771
Cheng C, Brookhart M. J Am Chem Soc, 2012, 134: 11304–11307
Das S, Wendt B, Möller K, Junge K, Beller M. Angew Chem Int Ed, 2012, 51: 1662–1666
Li B, Sortais JB, Darcel C. Chem Commun, 2013, 49: 3691–3693
Reeves JT, Tan Z, Marsini MA, Han ZS, Xu Y, Reeves DC, Lee H, Lu BZ, Senanayake CH. Adv Synth Catal, 2013, 355: 47–52
Blondiaux E, Cantat T. Chem Commun, 2014, 50: 9349–9352
Simmons BJ, Hoffmann M, Hwang J, Jackl MK, Garg NK. Org Lett, 2017, 19: 1910–1913
Igarashi M, Fuchikami T. Tetrahedron Lett, 2001, 42: 1945–1947
Tinnis F, Volkov A, Slagbrand T, Adolfsson H. Angew Chem Int Ed, 2016, 55: 4562–4566
Das S, Karmakar H, Bhattacharjee J, Panda TK. Dalton Trans, 2019, 48: 11978–11984
Leischner T, Artús Suarez L, Spannenberg A, Junge K, Nova A, Beller M. Chem Sci, 2019, 10: 10566–10576
Ong DY, Yen Z, Yoshii A, Revillo Imbernon J, Takita R, Chiba S. Angew Chem Int Ed, 2019, 58: 4992–4997
Sorribes I, Lemos SCS, Martín S, Mayoral A, Lima RC, Andrés J. Catal Sci Technol, 2019, 9: 6965–6976
Bisai MK, Gour K, Das T, Vanka K, Sen SS. Dalton Trans, 2021, 50: 2354–2358
Szostak M, Spain M, Eberhart AJ, Procter DJ. J Am Chem Soc, 2014, 136: 2268–2271
Lampland NL, Hovey M, Mukherjee D, Sadow AD. ACS Catal, 2015, 5: 4219–4226
Barger CJ, Dicken RD, Weidner VL, Motta A, Lohr TL, Marks TJ. J Am Chem Soc, 2020, 142: 8019–8028
Ye P, Shao Y, Ye X, Zhang F, Li R, Sun J, Xu B, Chen J. Org Lett, 2020, 22: 1306–1310
Tsutsumi H, Sunada Y, Nagashima H. Chem Commun, 2011, 47: 6581–6583
Du MH, Chen LQ, Jiang LP, Liu WD, Long LS, Zheng L, Kong XJ. J Am Chem Soc, 2022, 144: 5653–5660
Maity A, Teets TS. Chem Rev, 2016, 116: 8873–8911
Hadebe SW, Robinson RS. Eur J Org Chem, 2006, 2006(21): 4898–4904
Brown HC, Heim P. J Am Chem Soc, 1964, 86: 3566–3567
Kornet MJ, Thio PA, Tan SI. J Org Chem, 1968, 33: 3637–3639
Harder S, Spielmann J. J Organomet Chem, 2012, 698: 7–14
Müller F, Trincado M, Pribanic B, Vogt M, Grützmacher H. J Organomet Chem, 2016, 821: 154–162
Bage AD, Hunt TA, Thomas SP. Org Lett, 2020, 22: 4107–4112
Acknowledgements
This work was supported by the National Natural Science Foundation of China (92161104, 21871224, 92161203, 21721001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at https://chem.scichina.com and https://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Electronic Supporting Information
Rights and permissions
About this article
Cite this article
Weng, ZZ., Chen, CL., Ye, LW. et al. Lanthanide-oxo clusters for efficient catalytic reduction of carboxamides. Sci. China Chem. 66, 443–448 (2023). https://doi.org/10.1007/s11426-022-1493-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1493-y