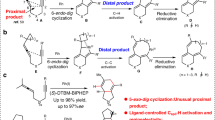
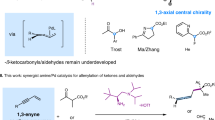
Abstract
We report the development of a new class of multifunctional chiral guanidine/Pd(0) catalyst system for 1,4-addition/arylation tandem reaction. A variety of tetra-substituted allenes were readily accessible from three-component “one-pot” transformations of acyclic or cyclic 2-activated 1,3-enynes, malonates and halobenzenes under mild reaction conditions. High levels of yield and enantioselectivity were achieved in the construction of stereogenic center and axis using readily available acyclic guanidine-amides. The mechanistic studies suggest that the guanidine/Pd(0) collaboration has obvious synergism to both base-dominated conjugate addition, and Pd(0)-dominated Heck-type reaction.

Similar content being viewed by others
References
For selected review, see: (a) Blieck R, Taillefer M, Monnier F. Chem Rev, 2020, 120: 13545–13598
Lu T, Lu Z, Ma ZX, Zhang Y, Hsung RP. Chem Rev, 2013, 113: 4862–4904
Alonso JM, Almendros P. Chem Rev, 2021, 121: 4193–4252
For selected examples, see: (a) Fu L, Greßies S, Chen P, Liu G. Chin J Chem, 2020, 38: 91–100
Bao X, Ren J, Yang Y, Ye X, Wang B, Wang H. Org Biomol Chem, 2020, 18: 7977–7986
Zhao JB, Xu YH. Asymmetric Synthesis of Chiral Allenes. Weinheim: Wiley-VCH, 2021. 141–172
Dherbassy Q, Manna S, Talbot FJT, Prasitwatcharakorn W, Perry GJP, Procter DJ. Chem Sci, 2020, 11: 11380–11393
Wu M, Han Z, Li K, Wu J’, Ding K, Lu Y. J Am Chem Soc, 2019, 141: 16362–16373
For selected examples, see: (a) Hashimoto T, Sakata K, Tamakuni F, Dutton MJ, Maruoka K. Nat Chem, 2013, 5: 240–244
Qian D, Wu LL, Lin Z, Sun J. Nat Commun, 2017, 8: 567–576
Zheng WF, Zhang W, Huang C, Wu P, Qian H, Wang L, Guo YL, Ma S. Nat Catal, 2019, 2: 997–1005
Chen M, Qian D, Sun J. Org Lett, 2019, 21: 8127–8131
Scheipers I, Mück-Lichtenfeld C, Studer A. Angew Chem Int Ed, 2019, 58: 6545–6548
Yang J, Wang Z, He Z, Li G, Hong L, Sun W, Wang R. Angew Chem Int Ed, 2020, 59: 642–647
Li F, Liang S, Luan Y, Chen X, Zhao H, Huang A, Li P, Li W. Org Chem Front, 2021, 8: 1243–1248
Schreib BS, Son M, Aouane FA, Baik MH, Carreira EM. J Am Chem Soc, 2021, 143: 21705–21712
For selected examples, see: (a) Qian H, Yu X, Zhang J, Sun J. J Am Chem Soc, 2013, 135: 18020–18023
Yao Q, Liao Y, Lin L, Lin X, Ji J, Liu X, Feng X. Angew Chem Int Ed, 2016, 55: 1859–1863
Poulsen PH, Li Y, Lauridsen VH, Jørgensen DKB, Palazzo TA, Meazza M, Jørgensen KA. Angew Chem Int Ed, 2018, 57: 10661–10665
Wang J, Zheng S, Rajkumar S, Xie J, Yu N, Peng Q, Yang X. Nat Commun, 2020, 11: 5527–5539
Hayashi T, Tokunaga N, Inoue K. Org Lett, 2004, 6: 305–307
Wang M, Liu ZL, Zhang X, Tian PP, Xu YH, Loh TP. J Am Chem Soc, 2015, 137: 14830–14833
Han JW, Tokunaga N, Hayashi T. J Am Chem Soc, 2001, 123: 12915–12916
Matsumoto Y, Naito M, Uozumi Y, Hayashi T. J Chem Soc Chem Commun, 1993, 1468–1469
Huang Y, del Pozo J, Torker S, Hoveyda AH. J Am Chem Soc, 2018, 140: 2643–2655
Yu S, Sang HL, Zhang SQ, Hong X, Ge S. Commun Chem, 2018, 1: 64–74
Adamson NJ, Jeddi H, Malcolmson SJ. J Am Chem Soc, 2019, 141: 8574–8583
Tsukamoto H, Konno T, Ito K, Doi T. Org Lett, 2019, 21: 6811–6814
Zhang W, Zheng S, Liu N, Werness JB, Guzei IA, Tang W. J Am Chem Soc, 2010, 132: 3664–3665
Yang SQ, Wang YF, Zhao WC, Lin GQ, He ZT. J Am Chem Soc, 2021, 143: 7285–7291
Ye J, Liao Y, Huang H, Liu Y, Fang D, Wang M, Hu L, Liao J. Chem Sci, 2021, 12: 3032–3038
For selected examples, see: (a) Liao Y, Yin X, Wang X, Yu W, Fang D, Hu L, Wang M, Liao J. Angew Chem Intl Edit, 2020, 59: 1176–1180
Dong X, Zhan T, Jiang S, Liu X, Ye L, Li Z, Gu Q, Liu X. Angew Chem Int Ed, 2021, 60: 2160–2164
Zeng Y, Chiou MF, Zhu X, Cao J, Lv D, Jian W, Li Y, Zhang X, Bao H. J Am Chem Soc, 2020, 142: 18014–18021
Law C, Kativhu E, Wang J, Morken JP. Angew Chem Int Ed, 2020, 59: 10311–10315
Tang Y, Chen Q, Liu X, Wang G, Lin L, Feng X. Angew Chem Int Ed, 2015, 54: 9512–9516
Liu Y, Liu X, Hu H, Guo J, Xia Y, Lin L, Feng X. Angew Chem Int Ed, 2016, 55: 4054–4058
Wang G, Liu X, Chen Y, Yang J, Li J, Lin L, Feng X. ACS Catal, 2016, 6: 2482–2486
Tang Y, Xu J, Yang J, Lin L, Feng X, Liu X. Chem, 2018, 4: 1658–1672
Xu X, Dong S, Feng LL, Wang S, Liu X, Feng X. Org Lett, 2020, 22: 2692–2696
Xiao Y, Zhang J. Angew Chem Int Ed, 2008, 47: 1903–1906
Xiao Y, Zhang J. Chem Commun, 2010, 46: 752–754
For selected examples, see: (a) Steuer L, Kaifer E, Himmel HJ. Dalton Trans, 2021, 50: 9467–9482
Bailey PJ, Pace S. Coord Chem Rev, 2001, 214: 91–141
Oakley SH, Coles MP, Hitchcock PB. Inorg Chem, 2004, 43: 7564–7566
Coles MP. Dalton Trans, 2006, 985
Elumalai P, Ujjval R, Nethaji M, Thirupathi N. Polyhedron, 2018, 151: 313–322
Cui XY, Tan CH, Leow D. Org Biomol Chem, 2019, 17: 4689–4699
Mishra V, Thomas JM, Chinnappan S, Thirupathi, N. J Organomet Chem, 2019, 892, 1–17
Francos J, Cadierno V. Dalton Trans, 2019, 48: 9021–9036
Zhang WX, Xu L, Xi Z. Chem Commun, 2015, 51: 254–265
Parker A, Lamata P, Viguri F, Rodríguez R, López JA, Lahoz FJ, García-Orduña P, Carmona D. Dalton Trans, 2020, 49: 13601–13617
Kim B, Chinn AJ, Fandrick DR, Senanayake CH, Singer RA, Miller SJ. J Am Chem Soc, 2016, 138: 7939–7945
Chinn AJ, Kim B, Kwon Y, Miller SJ. J Am Chem Soc, 2017, 139: 18107–18114
Kwon Y, Chinn AJ, Kim B, Miller SJ. Angew Chem Int Ed, 2018, 57: 6251–6255
Bárta O, Císařová I, Štěpnička P. Dalton Trans, 2021, 50: 14662–14671
Leitner Z, Císařová I, Štěpnička P. New J Chem, 2022, 46: 1060–1071
Zhu Y, Liu X, Dong S, Zhou Y, Li W, Lin L, Feng X. Angew Chem Int Ed, 2014, 53: 1636–1640
Chen Q, Tang Y, Huang T, Liu X, Lin L, Feng X. Angew Chem Int Ed, 2016, 55: 5286–5289
Chen Q, Xie L, Li Z, Tang Y, Zhao P, Lin L, Feng X, Liu X. Chem Commun, 2018, 54: 678–681
Ruan S, Zhong X, Chen Q, Feng X, Liu X. Chem Commun, 2020, 56: 2155–2158
Guo S, Dong P, Chen Y, Feng X, Liu X. Angew Chem Int Ed, 2018, 57: 16852–16856
Xie J, Liang R, Jia Y. Chin J Chem, 2021, 39: 710–728
Deposition Number 2129129 (D10) and 2129128 (D69) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
For selected examples, see: (a) Dong S, Feng X, Liu X. Chem Soc Rev, 2018, 47: 8525–8540
Dong S, Liu X, Zhu Y, He P, Lin L, Feng X. J Am Chem Soc, 2013, 135: 10026–10029
Li J, Mo Y, Yan L, Feng X, Su Z, Liu X. CCS Chem, 2022, 4: 650–659
For selected examples, see: (a) For selected examples, see: Li SH, Xie HB, Zhang SB, Lin YJ, Xu JN, Cao JG. Synlett, 2005, 12: 1885–1888
Li S, Lin Y, Xie H, Zhang S, Xu J. Org Lett, 2006, 8: 391–394
Li S, Lin Y, Cao J, Zhang S. J Org Chem, 2007, 72: 4067–4072
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21625205) and the Sichuan University (2020SCUNL204). We thank for Dr. Bo Gao (Sichuan University) for the help with the mass spectrometry and Dr. Yuqiao Zhou (Sichuan University) for the assistance with X-ray analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at https://chem.scichina.com and https://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wu, J., Ning, L. et al. Enantioselective synthesis of tetrasubstituted allenes via addition/arylation tandem reaction of 2-activated 1,3-enynes. Sci. China Chem. 66, 526–533 (2023). https://doi.org/10.1007/s11426-022-1443-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1443-5