Abstract
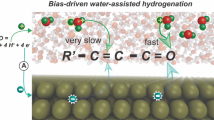
Electrochemical hydrogenation (ECH) of furfural, which uses the proton from water and avoids the usage of gaseous hydrogen and high pressure, is an efficient way to utilize biomass energy. Cu-based catalysts are promising catalysts for the ECH of furfural. However, their active sites and reaction mechanism have not been fully understood yet. This work unveils the active oxidation state of Cu-based electrocatalysts for the ECH of furfural. The co-existence of Cu+ and Cu0 on the CuO surface under the working potential is confirmed by a series of in situ characterizations. The poisoning experiment shows that the performance decreased heavily after the Cu+ was complexed with SCN−, indicating the decisive role of Cu+. Finally, the density functional theory (DFT) calculation suggests that the Cu0−Cu+ synergistic effect is beneficial to both kinetics and thermodynamics: Cu+ accelerates the second step hydrogenation process of furfural, and Cu0 reduces the energy barrier for the desorption of furfuryl alcohol. This work demonstrates the synergistic effect of Cu0 and Cu+ states for the electrochemical hydrogenation of furfural and provides a deeper understanding of the furfural hydrogenation mechanism.

Similar content being viewed by others
References
Akhade SA, Singh N, Gutiérrez OY, Lopez-Ruiz J, Wang H, Holladay JD, Liu Y, Karkamkar A, Weber RS, Padmaperuma AB, Lee MS, Whyatt GA, Elliott M, Holladay JE, Male JL, Lercher JA, Rousseau R, Glezakou VA. Chem Rev, 2020, 120: 11370–11419
Chen S, Wojcieszak R, Dumeignil F, Marceau E, Royer S. Chem Rev, 2018, 118: 11023–11117
Mariscal R, Maireles-Torres P, Ojeda M, Sádaba I, López Granados M. Energy Environ Sci, 2016, 9: 1144–1189
May AS, Biddinger EJ. ACS Catal, 2020, 10: 3212–3221
Zhou L, Zhu X, Su H, Lin H, Lyu Y, Zhao X, Chen C, Zhang N, Xie C, Li Y, Lu Y, Zheng J, Johannessen B, Jiang SP, Liu Q, Li Y, Zou Y, Wang S. Sci China Chem, 2021, 64: 1586–1595
Xu C, Paone E, Rodríguez-Padrón D, Luque R, Mauriello F. Chem Soc Rev, 2020, 49: 4273–4306
Lucas FWS, Grim RG, Tacey SA, Downes CA, Hasse J, Roman AM, Farberow CA, Schaidle JA, Holewinski A. ACS Energy Lett, 2021, 6: 1205–1270
Zhang X, Han M, Liu G, Wang G, Zhang Y, Zhang H, Zhao H. Appl Catal B-Environ, 2019, 244: 899–908
Carroll KJ, Burger T, Langenegger L, Chavez S, Hunt ST, Román-Leshkov Y, Brushett FR. ChemSusChem, 2016, 9: 1904–1910
Liu L, Liu H, Huang W, He Y, Zhang W, Wang C, Lin H. J Electroanal Chem, 2017, 804: 248–253
Nilges P, Schröder U. Energy Environ Sci, 2013, 6: 2925–2931
Chadderdon XH, Chadderdon DJ, Matthiesen JE, Qiu Y, Carraher JM, Tessonnier JP, Li W. J Am Chem Soc, 2017, 139: 14120–14128
Zhou P, Chen Y, Luan P, Zhang X, Yuan Z, Guo SX, Gu Q, Johannessen B, Mollah M, Chaffee AL, Turner DR, Zhang J. Green Chem, 2021, 23: 3028–3038
Solanki BS, Rode CV. Green Chem, 2019, 21: 6390–6406
Geng W, Li W, Liu L, Liu J, Liu L, Kong X. Fuel, 2020, 259: 116267
Lee J, Seo JH, Nguyen-Huy C, Yang E, Lee JG, Lee H, Jang EJ, Kwak JH, Lee JH, Lee H, An K. Appl Catal B-Environ, 2021, 282: 119576
Wang J, Tan HY, Zhu Y, Chu H, Chen HM. Angew Chem Int Ed, 2021, 60: 17254–17267
Rajendran K, Pandurangan N, Vinod CP, Khan TS, Gupta S, Haider MA, Jagadeesan D. Appl Catal B-Environ, 2021, 297: 120417
Min S, Yang X, Lu AY, Tseng CC, Hedhili MN, Li LJ, Huang KW. Nano Energy, 2016, 27: 121–129
Wei X, Li Y, Chen L, Shi J. Angew Chem Int Ed, 2021, 60: 3148–3155
Wang Y, Zhou W, Jia R, Yu Y, Zhang B. Angew Chem Int Ed, 2020, 59: 5350–5354
Chen X, Liu G, Zheng W, Feng W, Cao W, Hu W, Hu PA. Adv Funct Mater, 2016, 26: 8537–8544
Zhou XZ, Deng CP, Su YC. J Alloys Compd, 2010, 491: 92–97
Abbaspour A, Khajehzadeh A, Ghaffarinejad A. J Electroanal Chem, 2009, 631: 52–57
Anantharaj S, Karthik PE, Noda S. Angew Chem Int Ed, 2021, 60: 23051–23067
Zhou Y, Che F, Liu M, Zou C, Liang Z, De Luna P, Yuan H, Li J, Wang Z, Xie H, Li H, Chen P, Bladt E, Quintero-Bermudez R, Sham TK, Bals S, Hofkens J, Sinton D, Chen G, Sargent EH. Nat Chem, 2018, 10: 974–980
De Luna P, Quintero-Bermudez R, Dinh CT, Ross MB, Bushuyev OS, Todorović P, Regier T, Kelley SO, Yang P, Sargent EH. Nat Catal, 2018, 1: 103–110
Weng Z, Wu Y, Wang M, Jiang J, Yang K, Huo S, Wang XF, Ma Q, Brudvig GW, Batista VS, Liang Y, Feng Z, Wang H. Nat Commun, 2018, 9: 415
Jung H, Lee SY, Lee CW, Cho MK, Won DH, Kim C, Oh HS, Min BK, Hwang YJ. J Am Chem Soc, 2019, 141: 4624–4633
Arán-Ais RM, Scholten F, Kunze S, Rizo R, Roldan Cuenya B. Nat Energy, 2020, 5: 317–325
Penneman RA, Jones LH. J Chem Phys, 1956, 24: 293–296
Hage W, Hallbrucker A, Mayer E. J Phys Chem, 1992, 96: 6488–6493
Li X, Zhou D, Hao H, Chen H, Weng Y, Bian H. J Phys Chem Lett, 2020, 11: 548–555
Aristizábal A, Contreras S, Barrabés N, Llorca J, Tichit D, Medina F. Appl Catal B-Environ, 2011, 110: 58–70
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0710000), the National Natural Science Foundation of China (22122901, 21902047, 21825201, U19A2017), the Provincial Natural Science Foundation of Hunan (2020JJ5045, 2021JJ20024, 2021RC3054).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Xia, Z., Li, Y., Wu, J. et al. Promoting the electrochemical hydrogenation of furfural by synergistic Cu0−Cu+ active sites. Sci. China Chem. 65, 2588–2595 (2022). https://doi.org/10.1007/s11426-022-1407-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1407-0