Abstract
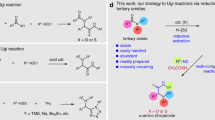
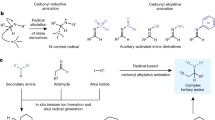
It is an unceasing goal for organic chemists to develop new catalytic methodologies for functional group transformations of widespread molecular structures. Amides are readily available from simple and reliable reactions, which are common structural units found in biologically active compounds. Consequently, they are attractive to be exploited in amine synthesis by reductive cross coupling. However, deoxygenative functionalization of amides is a long-standing challenge owing to the inertness of the resonance-stabilized amide C=O bond. In this work, a deoxygenative alkylation strategy was demonstrated, which combines amides and alkyl iodides to build structurally diverse tertiary alkylamines in a single step. Compared with previous deoxygenative alkylation of amides using organometallic reagents as functional partner, this work uses stable and easily available alkyl halides as functionalization reagents. The versatile and flexible strategy plus structural and functional diversity of readily available amides and alkyl iodides renders it highly appealing for the streamlined synthesis of tertiary amines and would be of much interest in areas such as pharmaceutical and agrochemical research.

Similar content being viewed by others
References
Smith AM, Whyman R. Chem Rev, 2014, 114: 5477–5510
Ruider SA, Maulide N. Angew Chem Int Ed, 2015, 54: 13856–13858
Volkov A, Tinnis F, Slagbrand T, Trillo P, Adolfsson H. Chem Soc Rev, 2016, 45: 6685–6697
Kaiser D, Bauer A, Lemmerer M, Maulide N. Chem Soc Rev, 2018, 47: 7899–7925
Huang PQ. Acta Chim Sin, 2018, 76: 357–365
Sato T, Yoritate M, Tajima H, Chida N. Org Biomol Chem, 2018, 16: 3864–3875
Cabrero-Antonino JR, Adam R, Papa V, Beller M. Nat Commun, 2020, 11: 3893–3910
Ong DY, Chen J, Chiba S. BCSJ, 2020, 93: 1339–1349
Hie L, Fine Nathel NF, Shah TK, Baker EL, Hong X, Yang YF, Liu P, Houk KN, Garg NK. Nature, 2015, 524: 79–83
Takise R, Muto K, Yamaguchi J. Chem Soc Rev, 2017, 46: 5864–5888
Li G, Ma S, Szostak M. Trends Chem, 2020, 2: 914–928
Bao CC, Luo YL, Du HZ, Guan BT. Sci China Chem, 2021, 64: 1349–1354
Xiao KJ, Luo JM, Ye KY, Wang Y, Huang PQ. Angew Chem Int Ed, 2010, 49: 3037–3040
Xiao KJ, Wang AE, Huang PQ. Angew Chem Int Ed, 2012, 51: 8314–8317
Kaiser D, Maulide N. J Org Chem, 2016, 81: 4421–4428
Schedler DJA, Godfrey AG, Ganem B. Tetrahedron Lett, 1993, 34: 5035–5038
Shirokane K, Kurosaki Y, Sato T, Chida N. Angew Chem Int Ed, 2010, 49: 6369–6372
Vincent G, Guillot R, Kouklovsky C. Angew Chem Int Ed, 2011, 50: 1350–1353
Seebach D. Angew Chem Int Ed, 2011, 50: 96–101
Pace V, Holzer W, Olofsson B. Adv Synth Catal, 2014, 356: 3697–3736
Ong DY, Fan D, Dixon DJ, Chiba S. Angew Chem Int Ed, 2020, 59: 11903–11907
Ou W, Huang PQ. Sci China Chem, 2019, 63: 11–15
Tahara A, Nagashima H. Tetrahedron Lett, 2020, 61: 151423–151430
Matheau-Raven D, Gabriel P, Leitch JA, Almehmadi YA, Yamazaki K, Dixon DJ. ACS Catal, 2020, 10: 8880–8897
Sunada Y, Kawakami H, Imaoka T, Motoyama Y, Nagashima H. Angew Chem Int Ed, 2009, 48: 9511–9514
Xie LG, Dixon DJ. Chem Sci, 2017, 8: 7492–7497
Rogova T, Gabriel P, Zavitsanou S, Leitch JA, Duarte F, Dixon DJ. ACS Catal, 2020, 10: 11438–11447
Matheau-Raven D, Dixon DJ. Angew Chem Int Ed, 2021, 60: 19725–19729
Gabriel P, Almehmadi YA, Wong ZR, Dixon DJ. J Am Chem Soc, 2021, 143: 10828–10835
Nakajima M, Sato T, Chida N. Org Lett, 2015, 17: 1696–1699
Katahara S, Kobayashi S, Fujita K, Matsumoto T, Sato T, Chida N. J Am Chem Soc, 2016, 138: 5246–5249
Takahashi Y, Sato T, Chida N. Chem Lett, 2019, 48: 1138–1141
Sugiyama Y, Soda Y, Yoritate M, Tajima H, Takahashi Y, Shibuya K, Ogihara C, Yokoyama T, Oishi T, Sato T, Chida N. Bull Chem Soc Jpn, 2022, 95: 278–287
Ou W, Han F, Hu XN, Chen H, Huang PQ. Angew Chem Int Ed, 2018, 57: 11354–11358
Chen DH, Sun WT, Zhu CJ, Lu GS, Wu DP, Wang AE, Huang PQ. Angew Chem Int Ed, 2021, 60: 8827–8831
Trillo P, Slagbrand T, Adolfsson H. Angew Chem Int Ed, 2018, 57: 12347–12351
Ronson TO, Renders E, Van Steijvoort BF, Wang X, Wybon CCD, Prokopcová H, Meerpoel L, Maes BUW. Angew Chem Int Ed, 2019, 58: 482–487
He Y, Wang X. Org Lett, 2021, 23: 225–230
Li Z, Zhao F, Ou W, Huang PQ, Wang X. Angew Chem Int Ed, 2021, 60: 26604–26609
Jiang F, Zhao F, He Y, Luo X, Wang X. Cell Rep Phys Sci, 2022: 100955
Wang XG, Ou W, Liu MH, Liu ZJ, Huang PQ. Org Chem Front, 2022, 9: 3237–3246
Crespi S, Fagnoni M. Chem Rev, 2020, 120: 9790–9833
Sumida Y, Ohmiya H. Chem Soc Rev, 2021, 50: 6320–6332
Latrache M, Hoffmann N. Chem Soc Rev, 2021, 50: 7418–7435
Xuan J, Xiao WJ. Angew Chem Int Ed, 2012, 51: 6828–6838
Shaw MH, Twilton J, MacMillan DWC. J Org Chem, 2016, 81: 6898–6926
Hopkinson MN, Sahoo B, Li JL, Glorius F. Chem Eur J, 2014, 20: 3874–3886
Skubi KL, Blum TR, Yoon TP. Chem Rev, 2016, 116: 10035–10074
Twilton J, Le C, Zhang P, Shaw MH, Evans RW, MacMillan DWC. Nat Rev Chem, 2017, 1: 0052
Friestad GK. Tetrahedron, 2001, 57: 5461–5496
Miyabe H, Yoshioka E, Kohtani S. COC, 2010, 14: 1254–1264
Friestad GK. Top Curr Chem, 2012, 320: 61–91
Tauber J, Imbri D, Opatz T. Molecules, 2014, 19: 16190–16222
Kumar R, Flodén NJ, Whitehurst WG, Gaunt MJ. Nature, 2020, 581: 415–420
Blackwell JH, Kumar R, Gaunt MJ. J Am Chem Soc, 2021, 143: 1598–1609
Chatgilialoglu C, Lalevée J. Molecules, 2012, 17: 527–555
Chatgilialoglu C, Ferreri C, Landais Y, Timokhin VI. Chem Rev, 2018, 118: 6516–6572
Dinnocenzo JP, Banach TE. J Am Chem Soc, 1989, 111: 8646–8653
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22171278, 21821002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Zhao, F., Jiang, F. & Wang, X. Deoxygenative alkylation of tertiary amides using alkyl iodides under visible light. Sci. China Chem. 65, 2231–2237 (2022). https://doi.org/10.1007/s11426-022-1331-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1331-y