Abstract
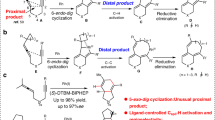
A chiral dirhodium complex is an effective and robust catalyst for asymmetric carbene transformations. However, dirhodium-catalyzed asymmetric ylide interception processes are rare, mainly because of the dissociation of the metal catalyst before the stereo-determining step. Herein, we report a chiral dirhodium(II)-catalyzed asymmetric annulation of vinyl diazoesters with α-hydroxyl ketones, which provides an efficient way to form chiral 2,3-dihydropyrans in good yields with excellent diastereos-electivities and enantioselectivities. This article is the first example of the chiral dirhodium complex—controlled asymmetric aldol-type interception of an in situ—formed oxonium ylide. The origin of the high stereoselectivity is well expounded via experimental and computational studies. These generated chiral products exhibit potent antiproliferation activity in three tested cancer cell lines, namely HCT116 (colon cancer), A549 (lung adenocarcinoma), and SJSA-1 (osteosarcoma cancer).

Similar content being viewed by others
References
Qi X, Lan Y. Acc Chem Res, 2021, 54: 2905–2915
Zhu D, Chen L, Fan H, Yao Q, Zhu S. Chem Soc Rev, 2020, 49: 908–950
Ford A, Miel H, Ring A, Slattery CN, Maguire AR, McKervey MA. Chem Rev, 2015, 115: 9981–10080
Davies HML, Denton JR. Chem Soc Rev, 2009, 38: 3061–3071
Padwa A. Chem Soc Rev, 2009, 38: 3072–3081
Kong L, Han X, Chen H, Sun H, Lan Y, Li X. ACS Catal, 2021, 11: 4929–4935
Davies HML, Liao K. Nat Rev Chem, 2019, 3: 347–360
Davies HML, Morton D. ACS Cent Sci, 2017, 3: 936–943
Pons A, Delion L, Poisson T, Charette AB, Jubault P. Acc Chem Res, 2021, 54: 2969–2990
Wang H, Guptill DM, Varela-Alvarez A, Musaev DG, Davies HML. Chem Sci, 2013, 4: 2844–2850
Xia Y, Qiu D, Wang J. Chem Rev, 2017, 117: 13810–13889
Zhang Y, Wang J. Coord Chem Rev, 2010, 254: 941–953
Nicolle SM, Lewis W, Hayes CJ, Moody CJ. Angew Chem Int Ed, 2015, 54: 8485–8489
Zhu SF, Zhou QL. Acc Chem Res, 2012, 45: 1365–1377
Zhu S. Chin J Chem, 2021, 39: 3211–3218
Suneja A, Loui HJ, Schneider C. Angew Chem Int Ed, 2020, 59: 5536–5540
Zhang D, Hu W. Chem Rec, 2017, 17: 739–753
Zhou CY, Wang JC, Wei J, Xu ZJ, Guo Z, Low KH, Che CM. Angew Chem, 2012, 124: 11538–11542
Liang XS, Li RD, Wang XC. Angew Chem Int Ed, 2019, 58: 13885–13889
Cheng QQ, Deng Y, Lankelma M, Doyle MP. Chem Soc Rev, 2017, 46: 5425–5443
Xu X, Doyle MP. Acc Chem Res, 2014, 47: 1396–1405
Dong K, Humeidi A, Griffith W, Arman H, Xu X, Doyle MP. Angew Chem Int Ed, 2021, 60: 13394–13400
Marichev KO, Wang K, Dong K, Greco N, Massey LA, Deng Y, Arman H, Doyle MP. Angew Chem, 2019, 131: 16334–16338
Deng Y, Massey LA, Zavalij PY, Doyle MP. Angew Chem Int Ed, 2017, 56: 7479–7483
Zhang B, Davies HML. Angew Chem Int Ed, 2020, 59: 4937–4941
Parr BT, Davies HML. Org Lett, 2015, 17: 794–797
Parr BT, Davies HML. Nat Commun, 2014, 5: 4455
Qin C, Davies HML. J Am Chem Soc, 2013, 135: 14516–14519
Parr BT, Li Z, Davies HML. Chem Sci, 2011, 2: 2378–2382
Yuan T, Ryckaert B, Van Hecke K, Hullaert J, Winne JM. Angew Chem Int Ed, 2021, 60: 4070–4074
Armengol-Relats H, Mato M, Echavarren AM. Angew Chem Int Ed, 2021, 60: 1916–1922
Wu R, Chen K, Ma J, Yu ZX, Zhu S. Sci China Chem, 2020, 63: 1230–1239
Bao M, Chen J, Pei C, Zhang S, Lei J, Hu W, Xu X. Sci China Chem, 2020, 64: 778–787
Dong G, Bao M, Xie X, Jia S, Hu W, Xu X. Angew Chem Int Ed, 2021, 60: 1992–1999
Reddy AGK, Niharika P, Zhou S, Jia SK, Shi T, Xu X, Qian Y, Hu W. Org Lett, 2020, 22: 2925–2930
Zhang D, Wang X, Zhang M, Kang Z, Xiao G, Xu X, Hu W. CCS Chem, 2020, 2: 432–439
Liu YC, Xiao S, Yang K, Ling L, Sun ZL, Liu ZY. J Mass Spectrom, 2017, 52: 378–396
Fu L, Huang X, Lai Z, Hu Y, Liu H, Cai X. Molecules, 2008, 13: 1923–1930
Krohn K, Dai J, Flörke U, Aust HJ, Dräger S, Schulz B. J Nat Prod, 2005, 68: 400–405
Timonen J, Vuolteenaho K, Leppänen T, Nieminen R, Moilanen E, Aulaskari P, Jänis J. J Heterocyclic Chem, 2015, 52: 1286–1295
Brito I, Dias T, Díaz-Marrero AR, Darias J, Cueto M. Tetrahedron, 2006, 62: 9655–9660
Zhu DX, Xia H, Liu JG, Chung LW, Xu MH. J Am Chem Soc, 2021, 143: 2608–2619
Liao K, Pickel TC, Boyarskikh V, Bacsa J, Musaev DG, Davies HML. Nature, 2017, 551: 609–613
Tsutsui H, Abe T, Nakamura S, Anada M, Hashimoto S. Chem Pharm Bull, 2005, 53: 1366–1368
CCDC 2060733 and 2069232 contain the supplementary crystallographic data for 4j and 4s, respectively. These data can be obtained free of charge from the Cambridge crystallographic data centre viawww.ccdc.cam.ac.uk/data_request/cif
Chung LW, Sameera WMC, Ramozzi R, Page AJ, Hatanaka M, Petrova GP, Harris TV, Li X, Ke Z, Liu F, Li HB, Ding L, Morokuma K. Chem Rev, 2015, 115: 5678–5796
Menikarachchi LC, Gascón JA. Curr Top Med Chem, 2010, 10: 46–54
Senn HM, Thiel W. Angew Chem Int Ed, 2009, 48: 1198–1229
Vreven T, Byun KS, Komáromi I, Dapprich S, Montgomery Jr. JA, Morokuma K, Frisch MJ. J Chem Theor Comput, 2006, 2: 815–826
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22001268, 21973113, 81973176), the Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery (2019B030301005), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06Y337) and the Guangdong Natural Science Fund (2020A1515010614), Key-Area Research and Development Program of Guangdong Province (2022B1111050003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The supporting information is available online at chem.scichina.com link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2022_1275_MOESM1_ESM.pdf
Chiral rhodium(II)-catalyzed asymmetric aldol-type interception of an oxonium ylide to assemble chiral 2,3-dihydropyrans
Rights and permissions
About this article
Cite this article
Xu, A., Zhou, X., Zheng, R. et al. Chiral rhodium(II)-catalyzed asymmetric aldol-type interception of an oxonium ylide to assemble chiral 2,3-dihydropyrans. Sci. China Chem. 65, 1607–1614 (2022). https://doi.org/10.1007/s11426-022-1275-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1275-9