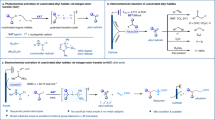
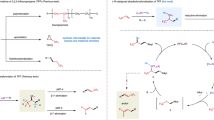
Abstract
Reported here is a precise electro-reduction strategy for radical defluorinative alkylation towards the synthesis of gem-difluoroalkenes from α-trifluoromethylstyrenes. According to the redox-potential difference of the radical precursors, direct or indirect electrolysis is respectively adopted to realize the precise reduction. An easy-to-handle, catalyst- and metal-free condition is developed for the reduction of alkyl radical precursors that are generally easier to be reduced than α-trifluoromethylstyrenes, while a novel electro-Ni-catalytic system is established for the electro-reduction of alkyl bromides or chlorides towards the electrochemical synthesis of gem-difluoroalkenes. The merit of this protocol is exhibited by its mild conditions, wide substrate scope, and scalable preparation. Mechanistic studies and DFT calculations proved that the coordination of α-trifluoromethylstyrenes to Ni-catalyst prevents the direct reduction of the alkene and, in turn, promotes the activation of alkyl bromide through halogen atom transfer mechanism.

Similar content being viewed by others
References
Yan M, Kawamata Y, Baran PS. Chem Rev, 2017, 117: 13230–13319
Wang H, Gao X, Lv Z, Abdelilah T, Lei A. Chem Rev, 2019, 119: 6769–6787
Jiang Y, Xu K, Zeng C. Chem Rev, 2018, 118: 4485–4540
Albert J F. Synthetic Organic Electrochemistry. New York: John Wiley & Sons, 1989. 339
Steckhan E. In: Organic Syntheses with Electrochemically Regenerable Redox Systems. Berlin, Heidelberg: Springer, 1987. 1–69
Francke R, Little RD. Chem Soc Rev, 2014, 43: 2492–2521
Siu JC, Fu N, Lin S. Acc Chem Res, 2020, 53: 547–560
Schmidt W, Steckhan E. Angew Chem Int Ed, 1978, 17: 673–674
Xiong P, Xu HC. Acc Chem Res, 2019, 52: 3339–3350
Ma C, Fang P, Liu ZR, Xu SS, Xu K, Cheng X, Lei A, Xu HC, Zeng C, Mei TS. Sci Bull, 2021, 66: 2412–2429
Jiao KJ, Xing YK, Yang QL, Qiu H, Mei TS. Acc Chem Res, 2020, 53: 300–310
Malapit CA, Prater MB, Cabrera-Pardo JR, Li M, Pham TD, McFadden TP, Blank S, Minteer SD. Chem Rev, 2022, 122: 3180–3218
Rafiee M, Miles KC, Stahl SS. J Am Chem Soc, 2015, 137: 14751–14757
Wang B, Peng P, Ma W, Liu Z, Huang C, Cao Y, Hu P, Qi X, Lu Q. J Am Chem Soc, 2021, 143: 12985–12991
Muller K, Faeh C, Diederich F. Science, 2007, 317: 1881–1886
Liu Q, Ni C, Hu J. Natl Sci Rev, 2017, 4: 303–325
Leriche C, He X, Chang CT, Liu H. J Am Chem Soc, 2003, 125: 6348–6349
Magueur G, Crousse B, Ourévitch M, Bonnet-Delpon D, Bégué JP. J Fluorine Chem, 2006, 127: 637–642
Pan Y, Qiu J, Silverman RB. J Med Chem, 2003, 46: 5292–5293
Ai HJ, Ma X, Song Q, Wu XF. Sci China Chem, 2021, 64: 1630–1659
Zhang X, Cao S. Tetrahedron Lett, 2017, 58: 375–392
Ni C, Hu J. Synthesis, 2014, 46: 842–863
Tian F, Yan G, Yu J. Chem Commun, 2019, 55: 13486–13505
Xing W, Wang J, Fu M, Fu Y. Chin J Chem, 2022, 40: 323–328
Kobayashi O, Uraguchi D, Yamakawa T. J Fluorine Chem, 2009, 130: 591–594
Corberán R, Mszar NW, Hoveyda AH. Angew Chem Int Ed, 2011, 50: 7079–7082
Dai W, Lin Y, Wan Y, Cao S. Org Chem Front, 2018, 5: 55–58
Cai Y, Zeng H, Zhu C, Liu C, Liu G, Jiang H. Org Chem Front, 2020, 7: 1260–1265
Lang SB, Wiles RJ, Kelly CB, Molander GA. Angew Chem Int Ed, 2017, 56: 15073–15077
Phelan JP, Lang SB, Sim J, Berritt S, Peat AJ, Billings K, Fan L, Molander GA. J Am Chem Soc, 2019, 141: 3723–3732
Zhang M, Zhang Z, He Y, Zou T, Qi Z, Fu Q, Wei J, Lu J, Wei S, Yi D. Adv Synth Catal, 2021, 363: 2110–2116
Xu W, Jiang H, Leng J, Ong HW, Wu J. Angew Chem Int Ed, 2020, 59: 4009–4016
Gao QS, Niu Z, Chen Y, Sun J, Han WY, Wang JY, Yu M, Zhou MD. Org Lett, 2021, 23: 6153–6157
Yue WJ, Day CS, Martin R. J Am Chem Soc, 2021, 143: 6395–6400
Guo YQ, Wu Y, Wang R, Song H, Liu Y, Wang Q. Org Lett, 2021, 23: 2353–2358
Li CY, Ma Y, Lei ZW, Hu XG. Org Lett, 2021, 23: 8899–8904
Chen F, Xu X, He Y, Huang G, Zhu S. Angew Chem Int Ed, 2020, 59: 5398–5402
Ding D, Lan Y, Lin Z, Wang C. Org Lett, 2019, 21: 2723–2730
Lin Z, Lan Y, Wang C. Org Lett, 2019, 21: 8316–8322
Yao C, Wang S, Norton J, Hammond M. J Am Chem Soc, 2020, 142: 4793–4799
Zhang C, Lin Z, Zhu Y, Wang C. J Am Chem Soc, 2021, 143: 11602–11610
Lu X, Wang XX, Gong TJ, Pi JJ, He SJ, Fu Y. Chem Sci, 2019, 10: 809–814
Lan Y, Yang F, Wang C. ACS Catal, 2018, 8: 9245–9251
Lu L, Li H, Zheng Y, Bu F, Lei A. CCS Chem, 2021, 3: 2669–2675
Gao XT, Zhang Z, Wang X, Tian JS, Xie SL, Zhou F, Zhou J. Chem Sci, 2020, 11: 10414–10420
Liu Y, Tao X, Mao Y, Yuan X, Qiu J, Kong L, Ni S, Guo K, Wang Y, Pan Y. Nat Commun, 2021, 12: 6745
Zhang H, Liang M, Zhang X, He MK, Yang C, Guo L, Xia W. Org Chem Front, 2021, 9: 95–101
Claraz A, Allain C, Masson G. Chem Eur J, 2022, 28: e202103337
Poutrel P, Pannecoucke X, Jubault P, Poisson T. Org Lett, 2020, 22: 4858–4863
Hu J, Yang Y, Lou Z, Ni C, Hu J. Chin J Chem, 2018, 36: 1202–1208
Ichitsuka T, Fujita T, Ichikawa J. ACS Catal, 2015, 5: 5947–5950
Ichitsuka T, Fujita T, Arita T, Ichikawa J. Angew Chem Int Ed, 2014, 53: 7564–7568
Guo L, Yuan M, Zhang Y, Wang F, Zhu S, Gutierrez O, Chu L. J Am Chem Soc, 2020, 142: 20390–20399
Yuan M, Song Z, Badir SO, Molander GA, Gutierrez O. J Am Chem Soc, 2020, 142: 7225–7234
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFA1500100), the National Natural Science Foundation of China (22031008) and the Science Foundation of Wuhan (2020010601012192). The theoretical calculations were performed on the supercomputing system in the Supercomputing Center of Wuhan University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yan, X., Wang, S., Liu, Z. et al. Precise electro-reduction of alkyl halides for radical defluorinative alkylation. Sci. China Chem. 65, 762–770 (2022). https://doi.org/10.1007/s11426-021-1210-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1210-y