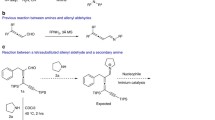
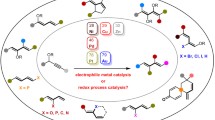
Abstract
The strategy toward the synthesis of various 1,3-dienals or 1,3-dienones is disclosed between diazo compounds and furans, which features metal-free, additive-free, broad functional group tolerance, and readily accessible starting materials. Notably, this strategy is applicable in both intramolecular and intermolecular protocols. Mechanistic studies suggested that the reactions undergo a cyclopropanation/rearrangement sequence. With an E/E-1,3-dienal, corresponding N-tosylhydrazones were readily prepared and subjected to phenylboronic acid to form a double bond migration product and indoles to construct a five-member ring via [3 + 2] annulation reaction.

Similar content being viewed by others
References
Inano H, Suzuki K, Ishii-Ohba H, Yamanouchi H, Takahashi M, Wakabayashi K. Carcinogenesis, 1993, 14: 2157–2163
Paik IH, Xie S, Shapiro TA, Labonte T, Narducci Sarjeant AA, Baege AC, Posner GH. J Med Chem, 2006, 49: 2731–2734
Reymond S, Cossy J. Chem Rev, 2008, 108: 5359–5406
Zhu Y, Cornwall RG, Du H, Zhao B, Shi Y. Acc Chem Res, 2014, 47: 3665–3678
Chen JR, Hu XQ, Lu LQ, Xiao WJ. Chem Rev, 2015, 115: 5301–5365
Heravi MM, Ahmadi T, Ghavidel M, Heidari B, Hamidi H. RSC Adv, 2015, 5: 101999–102075
Büschleb M, Dorich S, Hanessian S, Tao D, Schenthal KB, Overman LE. Angew Chem Int Ed, 2016, 55: 4156–4186
Diels O, Alder K. Justus Liebigs Ann Chem, 1928, 460: 98–122
Bradley AZ, Johnson RP. J Am Chem Soc, 1997, 119: 9917–9918
Du H, Zhao B, Shi Y. J Am Chem Soc, 2007, 129: 762–763
Yang XH, Dong VM. J Am Chem Soc, 2017, 139: 1774–1777
Nie SZ, Davison RT, Dong VM. J Am Chem Soc, 2018, 140: 16450–16454
Chen XW, Zhu L, Gui YY, Jing K, Jiang YX, Bo ZY, Lan Y, Li J, Yu DG. J Am Chem Soc, 2019, 141: 18825–18835
Zhang Q, Dong D, Zi W. J Am Chem Soc, 2020, 142: 15860–15869
Zhang Z, Xiao F, Wu HM, Dong XQ, Wang CJ. Org Lett, 2020, 22: 569–574
Yoo KS, Yoon CH, Mishra RK, Jung YC, Yi SW, Jung KW. J Am Chem Soc, 2006, 128: 16384–16393
Liu Q, Wang ZY, Peng XS, Wong HNC. J Org Chem, 2018, 83: 6325–6333
Nguyen VT, Dang HT, Pham HH, Nguyen VD, Flores-Hansen C, Arman HD, Larionov OV. J Am Chem Soc, 2018, 140: 8434–8438
Lv L, Zhu D, Qiu Z, Li J, Li CJ. ACS Catal, 2019, 9: 9199–9205
Hamaguchi T, Takahashi Y, Tsuji H, Kawatsura M. Org Lett, 2020, 22: 1124–1129
Wang GW, Boyd O, Young TA, Bertrand SM, Bower JF. J Am Chem Soc, 2020, 142: 1740–1745
Mehta G, Rao HSP. Synthesis of conjugated dienes and polyenes. Secondary Synthesis of conjugated dienes and polyenes, 1997, 359–480
Szliszka E, Czuba Z, Domino M, Mazur B, Zydowicz G, Krol W. Molecules, 2009, 14: 738–754
De Paolis M, Chataigner I, Maddaluno J. Recent advances in stereoselective synthesis of 1,3-dienes. Secondary Recent advances in stereoselective synthesis of 1,3-dienes, 2012,87–146
Gigant N, Bäckvall JE. Chem Eur J, 2013, 19: 10799–10803
Feng R, Yu W, Wang K, Liu Z, Zhang Y. Adv Synth Catal, 2014, 356: 1501–1508
Hu XH, Zhang J, Yang XF, Xu YH, Loh TP. J Am Chem Soc, 2015, 137: 3169–3172
Zhao Q, Tognetti V, Joubert L, Besset T, Pannecoucke X, Bouillon JP, Poisson T. Org Lett, 2017, 19: 2106–2109
Zhao Q, Wang J, Besset T, Pannecoucke X, Bouillon JP, Poisson T. Tetrahedron, 2018, 74: 6033–6040
Meng K, Sun Y, Zhang J, Zhang K, Ji X, Ding L, Zhong G. Org Lett, 2019, 21: 8219–8224
Wang YC, Huang YH, Tsai HC, Basha RS, Chou CM. Org Lett, 2020, 22: 6765–6770
Dethe DH, Beeralingappa NC, Das S, Nirpal AK. Chem Sci, 2021, 12: 4367–4372
Li Y, Hao M, Chang Y-, Liu Y, Wang W-, Sun N, Zhu W-, Gao Z. Chin J Chem, 2021, 39: 2962–2966
Wang H, Gu S, Yan Q, Ding L, Chen FE. Green Synthesis Catal, 2020, 1: 12–25
Hu TJ, Li MY, Zhao Q, Feng CG, Lin GQ. Angew Chem Int Ed, 2018, 57: 5871–5875
Al-Jawaheri Y, Kimber MC. Org Lett, 2016, 18: 3502–3505
Wang J, Dong Z, Yang C, Dong G. Nat Chem, 2019, 11: 1106–1112
Ma X, Li P, Liang J, An H, Yang K, Song Q. Cell Rep Phys Sci, 2021, 2: 100629
Flynn AB, Ogilvie WW. Chem Rev, 2007, 107: 4698–4745
Deagostino A, Prandi C, Tabasso S, Venturello P. Molecules, 2010, 15: 2667–2685
Kuang Z, Gao G, Song Q. Sci China Chem, 2018, 62: 62–66
Xiang L, Yang K, Song QL. Chin Chem Lett, 2017, 28: 517–520
Smaldone RA, Thompson CM, Evans M, Voit W. Nat Chem, 2017, 9: 97–102
Xia Y, Qiu D, Wang J. Chem Rev, 2017, 117: 13810–13889
Zhang Z, Sheng Z, Yu W, Wu G, Zhang R, Chu WD, Zhang Y, Wang J. Nat Chem, 2017, 9: 970–976
Doyle MP, Forbes DC. Chem Rev, 1998, 98: 911–936
Doyle MP, Duffy R, Ratnikov M, Zhou L. Chem Rev, 2010, 110: 704–724
Xu X, Doyle MP. Acc Chem Res, 2014, 47: 1396–1405
Cheng QQ, Yedoyan J, Arman H, Doyle MP. J Am Chem Soc, 2016, 138: 44–47
Cheng QQ, Yedoyan J, Arman H, Doyle MP. Angew Chem Int Ed, 2016, 55: 5573–5576
Qiu H, Srinivas HD, Zavalij PY, Doyle MP. J Am Chem Soc, 2016, 138: 1808–1811
Deng Y, Massey LA, Rodriguez Núñez YA, Arman H, Doyle MP. Angew Chem Int Ed, 2017, 56: 12292–12296
Duan A, Yu P, Liu F, Qiu H, Gu FL, Doyle MP, Houk KN. J Am Chem Soc, 2017, 139: 2766–2770
Davies HML, Beckwith REJ. Chem Rev, 2003, 103: 2861–2904
Davies HML, Manning JR. Nature, 2008, 451: 417–424
Davies HML, Morton D. Chem Soc Rev, 2011, 40: 1857–1869
Briones JF, Davies HML. J Am Chem Soc, 2012, 134: 11916–11919
Guzmán PE, Lian Y, Davies HML. Angew Chem Int Ed, 2014, 53: 13083–13087
Fu L, Guptill DM, Davies HML. J Am Chem Soc, 2016, 138: 5761–5764
Wei B, Sharland JC, Lin P, Wilkerson-Hill SM, Fullilove FA, McKinnon S, Blackmond DG, Davies HML. ACS Catal, 2020, 10: 1161–1170
Zhang B, Davies HML. Angew Chem Int Ed, 2020, 59: 4937–4941
Taniguchi H, Ohmura T, Suginome M. J Am Chem Soc, 2009, 131: 11298–11299
Hansmann MM, Melen RL, Rudolph M, Rominger F, Wadepohl H, Stephan DW, Hashmi ASK. J Am Chem Soc, 2015, 137: 15469–15477
Pitts CR, Ling B, Snyder JA, Bragg AE, Lectka T. J Am Chem Soc, 2016, 138: 6598–6609
Mai S, Song Q. Angew Chem Int Ed, 2017, 56: 7952–7957
Garve LKB, Jones PG, Werz DB. Angew Chem Int Ed, 2017, 56: 9226–9230
Rao C, Mai S, Song Q. Chem Commun, 2018, 54: 5964–5967
Guo Y, Nguyen TV, Koenigs RM. Org Lett, 2019, 21: 8814–8818
Li ML, Yu JH, Li YH, Zhu SF, Zhou QL. Science, 2019, 366: 990–994
Sullivan RJ, Freure GPR, Newman SG. ACS Catal, 2019, 9: 5623–5630
Ge L, Wang DX, Xing R, Ma D, Walsh PJ, Feng C. Nat Commun, 2019, 10: 4367
Luo W, Sun Z, Fernando EHN, Nesterov VN, Cundari TR, Wang H. ACS Catal, 2019, 9: 8285–8293
Mills LR, Monteith JJ, Dos Passos Gomes G, Aspuru-Guzik A, Rousseaux SAL. J Am Chem Soc, 2020, 142: 13246–13254
Augustin AU, Werz DB. Acc Chem Res, 2021, 54: 1528–1541
Guo L, Noble A, Aggarwal VK. Angew Chem Int Ed, 2021, 60: 212–216
Zuo Z, Daniliuc CG, Studer A. Angew Chem Int Ed, 2021, 60: 25252–25257
Zhao P, Li Z, He J, Liu X, Feng X. Sci China Chem, 2021, 64: 1355–1360
Muizebelt WJ, Nivard RJF. J Chem Soc B, 1968, 913
Benson SW, Egger KW, Golden DM. J Am Chem Soc, 2002, 87: 468–476
Dickinson RG, Lotzkar H. J Am Chem Soc, 2002, 59: 472–475
Hepperle SS, Li Q, East ALL. J Phys Chem A, 2005, 109: 10975–10981
Lian Y, Miller LC, Born S, Sarpong R, Davies HML. J Am Chem Soc, 2010, 132: 12422–12425
Zhou L, Ma J, Zhang Y, Wang J. Tetrahedron Lett, 2011, 52: 5484–5487
Harrar K, Reiser O. Chem Commun, 2012, 48: 3457–3459
Bloch R, Hassan D, Mandard X. Tetrahedron Lett, 1983, 24: 4691–4694
CCDC numbers 1942363, 1942345. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
Singh TP, Singh OM. MRMC, 2017, 18
Rakhit A, Hurley ME, Tipnis V, Coleman J, Rommel A, Brunner HR. J Clin Pharmacol, 1986, 26: 156–164
Kochanowska-Karamyan AJ, Hamann MT. Chem Rev, 2010, 110: 4489–4497
Ishikura M, Abe T, Choshi T, Hibino S. Nat Prod Rep, 2013, 30: 694–752
Barluenga J, Moriel P, Valdés C, Aznar F. Angew Chem Int Ed, 2007, 46: 5587–5590
Zhou YG. Acc Chem Res, 2007, 40: 1357–1366
Gilmore CD, Allan KM, Stoltz BM. J Am Chem Soc, 2008, 130: 1558–1559
Wang DS, Chen QA, Lu SM, Zhou YG. Chem Rev, 2012, 112: 2557–2590
Ascic E, Buchwald SL. J Am Chem Soc, 2015, 137: 4666–4669
Panda S, Ready JM. J Am Chem Soc, 2018, 140: 13242–13252
Cheng YZ, Zhao QR, Zhang X, You SL. Angew Chem Int Ed, 2019, 58: 18069–18074
Zhang HJ, Gu Q, You SL. Org Lett, 2019, 21: 9420–9424
Yang K, Lou Y, Wang C, Qi LW, Fang T, Zhang F, Xu H, Zhou L, Li W, Zhang G, Yu P, Song Q. Angew Chem Int Ed, 2020, 59: 3294–3299
Acknowledgements
This work was supported by the National Natural Science Foundation (21772046, 21931013) and Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University. The authors also thank the Instrumental Analysis Center of Huaqiao University for analysis support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Electronic Supplementary Information For
Rights and permissions
About this article
Cite this article
Feng, Q., Wang, S., Ma, X. et al. Design, synthesis, and applications of stereospecific 1,3-diene carbonyls. Sci. China Chem. 65, 912–917 (2022). https://doi.org/10.1007/s11426-021-1204-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1204-5