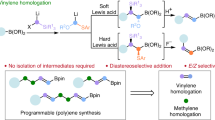
Abstract
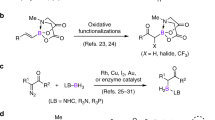
The semipinacol rearrangement is one of the classic yet useful synthetic tools in organic synthesis. However, semipinacol rearrangements involving heteroatom-migration are rare. Reported herein is a boryl-migratory semipinacol rearrangement of α-hydroxyallylboronates and α-hydroxypropargylboronates triggered by diverse halogen-, oxygen-, sulfur- and selenium-containing electrophiles. The protocol leads to a mild and facile access to organoborons bearing valuable functionalities. The σ (C—B) hyperconjugation is believed to be the key factor that leads to the observed exclusive chemoselectivity and enhanced reactivity. Synthetic utilities of the formed products were demonstrated.

Similar content being viewed by others
References
Song ZL, Fan CA, Tu YQ. Chem Rev, 2011, 111: 7523–7556
Wang B, Tu YQ. Acc Chem Res, 2011, 44: 1207–1222
Wang S-H, Tu Y-Q, Tang M. The semipinacol rearrangements. In: Knochel P, Molander GA, Eds. Comprehensive Organic Synthesis. 2nd Ed. Amsterdam: Elsevier, 2014. 795–852
Nakamura K, Osamura Y. J Am Chem Soc, 1993, 115: 9112–9120
Shubin VG. Rearrangements of carbocations by 1,2-shifts. In: Rees Ch, Ed. Contemporary Problems in Carbonium Ion Chemistry I/II. Topics in Current Chemistry. Berlin, Heidelberg: Springer, 1984. 267–341
Winstein S, Lindegren CR, Marshall H, Ingraham LL. J Am Chem Soc, 1953, 75: 147–155
Billamboz M, Banaszak E, Rigo B. ChemistrySelect, 2018, 3: 10236–10243
Gutiérrez-Bonet Á, Flores-Gaspar A, Martin R. J Am Chem Soc, 2013, 135: 12576–12579
Stopka T, Schröder S, Maulide N, Niggemann M. Tetrahedron, 2020, 76: 131460
Robiette R, Fang GY, Harvey JN, Aggarwal VK. Chem Commun, 2006, 741
Rooke DA, Ferreira EM. J Am Chem Soc, 2010, 132: 11926–11928
Barczak NT, Rooke DA, Menard ZA, Ferreira EM. Angew Chem Int Ed, 2013, 52: 7579–7582
Tan DH, Cai YH, Zeng YF, Lv WX, Yang L, Li Q, Wang H. Angew Chem Int Ed, 2019, 58: 13784–13788
Lv WX, Zeng YF, Li Q, Chen Y, Tan DH, Yang L, Wang H. Angew Chem Int Ed, 2016, 55: 10069–10073
Zeng YF, Ji WW, Lv WX, Chen Y, Tan DH, Li Q, Wang H. Angew Chem Int Ed, 2017, 56: 14707–14711
Lv WX, Li Q, Li JL, Li Z, Lin E, Tan DH, Cai YH, Fan WX, Wang H. Angew Chem Int Ed, 2018, 57: 16544–16548
Yang L, Tan DH, Fan WX, Liu XG, Wu JQ, Huang ZS, Li Q, Wang H. Angew Chem Int Ed, 2021, 60: 3454–3458
Zeng YF, Liu XG, Tan DH, Fan WX, Li YN, Guo Y, Wang H. Chem Commun, 2020, 56: 4332–4335
Wierschke SG, Chandrasekhar J, Jorgensen WL. J Am Chem Soc, 1985, 107: 1496–1500
Lambert JB, Zhao Y, Emblidge RW, Salvador LA, Liu X, So JH, Chelius EC. Acc Chem Res, 1999, 32: 183–190
Beļaunieks R, Puriņš M, Turks M. Synthesis, 2020, 52: 2147–2161
Dai W, Geib SJ, Curran DP. J Am Chem Soc, 2019, 141: 12355–12361
Yamashita M, Suzuki Y, Segawa Y, Nozaki K. JAm Chem Soc, 2007, 129: 9570–9571
Molander GA, Raushel J, Ellis NM. J Org Chem, 2010, 75: 4304–4306
He Z, Trinchera P, Adachi S, St. Denis JD, Yudin AK. Angew Chem Int Ed, 2012, 51: 11092–11096
Scharnagl FK, Bose SK, Marder TB. Org Biomol Chem, 2017, 15: 1738–1752
Taguchi J, Ikeda T, Takahashi R, Sasaki I, Ogasawara Y, Dairi T, Kato N, Yamamoto Y, Bode JW, Ito H. Angew Chem Int Ed, 2017, 56: 13847–13851
Lepage ML, Lai S, Peressin N, Hadjerci R, Patrick BO, Perrin DM. Angew Chem Int Ed, 2017, 56: 15257–15261
Wu D, Taguchi J, Tanriver M, Bode JW. Angew Chem Int Ed, 2020, 59: 16847–16858
Šterman A, Sosič I, Gobec S, Časar Z. ACS Omega, 2020, 5: 17868–17875
Cheng LJ, Zhao S, Mankad NP. Angew Chem, 2021, 133: 2122–2126
Schuhmacher A, Ryan SJ, Bode JW. Angew Chem Int Ed, 2021, 60: 3918–3922
Ivon YM, Mazurenko IV, Kuchkovska YO, Voitenko ZV, Grygorenko OO. Angew Chem Int Ed, 2020, 59: 18016–18022
Kisu H, Sakaino H, Ito F, Yamashita M, Nozaki K. J Am Chem Soc, 2016, 138: 3548–3552
Jana K, Bhunia A, Studer A. Chem, 2020, 6: 512–522
Wang D, Mück-Lichtenfeld C, Studer A. J Am Chem Soc, 2020, 142: 9119–9123
Lee CF, Diaz DB, Holownia A, Kaldas SJ, Liew SK, Garrett GE, Dudding T, Yudin AK. Nat Chem, 2018, 10: 1062–1070
He Z, Yudin AK. J Am Chem Soc, 2011, 133: 13770–13773
Li J, Burke MD. J Am Chem Soc, 2011, 133: 13774–13777
Shiroodi RK, Koleda O, Gevorgyan V. J Am Chem Soc, 2014, 136: 13146–13149
Shono T, Fujita K, Kumai S, Watanabe T, Nishiguchi I. Tetrahedron Lett, 1972, 13: 3249–3252
Shimazaki M, Hara H, Suzuki K. Tetrahedron Lett, 1989, 30: 5443–5446
Zang W, Wei Y, Shi M. Org Lett, 2020, 22: 5466–5472
Nakamura K, Osamura Y. Tetrahedron Lett, 1990, 31: 251–254
Snape TJ. Chem Soc Rev, 2007, 36: 1823–1842
Bégué JP, Bonnet-Delpon D, Crousse B. Synlett, 2004, 18–29
Vincenzi M, Mercurio FA, Leone M. Curr Protein Pept Sci, 2019, 20: 425–451
Ansorge A, Brauer DJ, Bürger H, Hagen T, Pawelke G. J Organomet Chem, 1993, 444: 5–14
Deloux L, Skrzypczak-Jankun E, Cheesman BV, Srebnik M, Sabat M. J Am Chem Soc, 1994, 116: 10302–10303
Zhu D, Ding TM, Luo HY, Ke H, Chen ZM. Org Lett, 2020, 22: 7699–7703
Wu P, Wu K, Wang L, Yu Z. Org Lett, 2017, 19: 5450–5453
Gillis EP, Burke MD. J Am Chem Soc, 2007, 129: 6716–6717
Li J, Ballmer SG, Gillis EP, Fujii S, Schmidt MJ, Palazzolo AME, Lehmann JW, Morehouse GF, Burke MD. Science, 2015, 347: 1221–1226
Li J, Grillo AS, Burke MD. Acc Chem Res, 2015, 48: 2297–2307
Lehmann JW, Blair DJ, Burke MD. Nat Rev Chem, 2018, 2: 0115
Sadhukhan S, Baire B. Chem Eur J, 2019, 25: 9816–9820
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22022114, 21971261), the Key Project of Chinese National Programs for Fundamental Research and Development (2016YFA0602900), the Guang-dong Basic and Applied Basic Research Foundation (2020A1515010624), the Fundamental Research Funds for the Central Universities (20ykzd12), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Y093), and the China Postdoctoral Science Foundation (2021M69360).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tan, DH., Chen, ZH., Yang, L. et al. A boryl-migratory semipinacol rearrangement. Sci. China Chem. 65, 746–752 (2022). https://doi.org/10.1007/s11426-021-1188-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1188-x