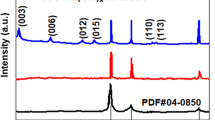
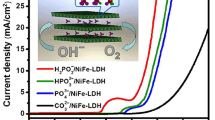
Abstract
Developing efficient catalysts with high durability and activity for the oxygen evolution reaction (OER) is imperative for sustainable energy conversion technologies, including hydrogen generation and CO2 reduction, as well as other electrochemical energy storage systems. To this end, a comprehensive understanding of the mechanism for the water oxidation reaction is vital. Herein, a surfactant, nonafluoro-1-butanesulfonate (FBS), was introduced into Ni-Fe layered double hydroxide (NiFe-FBS/CFP) via electrochemical deposition on the surface of a carbon fiber paper (CFP) substrate. The as-prepared NiFe-FBS/CFP electrode exhibited excellent catalytic activities for OER compared to the Ni-Fe layered double hydroxide based electrode (NiFe-LDH/CFP), an excellent stability of 15 h, and an ultralow Tafel slope of 25.8 mV dec−1. Furthermore, by combining the results of pH-dependent kinetics investigations, chemical probing, proton inventory studies, and isotopic and atom-protontransfer measurements, it was observed that a proton-transfer process controls the reaction rates of both the NiFe-LDH and NiFe-FBS catalysts, and the residual sulfonate groups serve as proton transfer mediator to accelerate the proton transfer rate.

Similar content being viewed by others
References
Dionigi F, Zeng Z, Sinev I, Merzdorf T, Deshpande S, Lopez MB, Kunze S, Zegkinoglou I, Sarodnik H, Fan D, Bergmann A, Drnec J, Araujo JF, Gliech M, Teschner D, Zhu J, Li WX, Greeley J, Cuenya BR, Strasser P. Nat Commun, 2020, 11: 2522
Li F, Yang H, Li W, Sun L. Joule, 2018, 2: 36–60
He J, Zou Y, Huang Y, Li C, Liu Y, Zhou L, Dong CL, Lu X, Wang S. Sci China Chem, 2020, 63: 1684–1693
Li H, Xie F, Zhang MT. ACS Catal, 2021, 11: 68–73
Meyer TJ, Huynh MHV, Thorp HH. Angew Chem Int Ed, 2007, 46: 5284–5304
Moreno-Hernandez IA, MacFarland CA, Read CG, Papadantonakis KM, Brunschwig BS, Lewis NS. Energy Environ Sci, 2017, 10: 2103–2108
Jiang C, Moniz SJA, Wang A, Zhang T, Tang J. Chem Soc Rev, 2017, 46: 4645–4660
Yang H, Gao S, Rao D, Zhang C, Zhou X, Yang S, Ye J, Yang S, Lai F, Yan X. Sci China Chem, 2021, 64: 101–108
Zhao WN, Liu ZP. Chem Sci, 2014, 5: 2256–2264
Zhang Y, Zhang H, Ji H, Ma W, Chen C, Zhao J. J Am Chem Soc, 2016, 138: 2705–2711
Savéant JM. Angew Chem Int Ed, 2019, 58: 2125–2128
Lee CH, Dogutan DK, Nocera DG. J Am Chem Soc, 2011, 133: 8775–8777
Liu Y, McCrory CCL. Nat Commun, 2019, 10: 1683
Bhunia S, Rana A, Roy P, Martin DJ, Pegis ML, Roy B, Dey A. J Am Chem Soc, 2018, 140: 9444–9457
Li W, Li F, Yang H, Wu X, Zhang P, Shan Y, Sun L. Nat Commun, 2019, 10: 5074
Devanathan R, Dupuis M. Phys Chem Chem Phys, 2012, 14: 11281
Lu Z, Xu W, Zhu W, Yang Q, Lei X, Liu J, Li Y, Sun X, Duan X. Chem Commun, 2014, 50: 6479–6482
Abellán G, Coronado E, Martí-Gastaldo C, Pinilla-Cienfuegos E, Ribera A. J Mater Chem, 2010, 20: 7451–7455
Wang Z, Wang J, Li Z, Gong P, Liu X, Zhang L, Ren J, Wang H, Yang S. Carbon, 2012, 50: 5403–5410
An H, Li Y, Feng Y, Cao Y, Cao C, Long P, Li S, Feng W. Chem Commun, 2018, 54: 2727–2730
Tressaud A, Moguet F, Flandrois S, Chambon M, Guimon C, Nanse G, Papirer E, Gupta V, Bahl OP. J Phys Chem Solids, 1996, 57: 745–751
Chen GF, Luo Y, Ding LX, Wang H. ACS Catal, 2018, 8: 526–530
Nasef MM, Saidi H, Nor HM, Yarmo MA. J Appl Polym Sci, 2000, 76: 336–349
Lv D, Li Y, Wang L. Int J Biol Macromolecules, 2020, 148: 979–987
Kim SS, Britcher L, Kumar S, Griesser HJ. JSM, 2018, 47: 1913–1922
Li J, Huang W, Wang M, Xi S, Meng J, Zhao K, Jin J, Xu W, Wang Z, Liu X, Chen Q, Xu L, Liao X, Jiang Y, Owusu KA, Jiang B, Chen C, Fan D, Zhou L, Mai L. ACS Energy Lett, 2019, 4: 285–292
Sun F, Wang G, Ding Y, Wang C, Yuan B, Lin Y. Adv Energy Mater, 2018, 8: 1800584
Tsai CE, Hwang BJ. Fuel Cells, 2007, 7: 408–416
Gruger A, Régis A, Schmatko T, Colomban P. Vibal Spectr, 2001, 26: 215–225
Qiu Z, Tai CW, Niklasson GA, Edvinsson T. Energy Environ Sci, 2019, 12: 572–581
Trześniewski BJ, Diaz-Morales O, Vermaas DA, Longo A, Bras W, Koper MTM, Smith WA. J Am Chem Soc, 2015, 137: 15112–15121
Edwards HGM, Brown DR, Dale JR, Plant S. J Mol Structure, 2001, 595: 111–125
Wang J, Zhong H, Wang Z, Meng F, Zhang X. ACS Nano, 2016, 10: 2342–2348
Mavrikis S, Perry SC, Leung PK, Wang L, Ponce de León C. ACS Sustain Chem Eng, 2020, 9: 76–91
Wei C, Xu ZJ. Small Methods, 2018, 2: 1800168
Masa J, Piontek S, Wilde P, Antoni H, Eckhard T, Chen YT, Muhler M, Apfel UP, Schuhmann W. Adv Energy Mater, 2019, 9: 1900796
Yang C, Fontaine O, Tarascon JM, Grimaud A. Angew Chem Int Ed, 2017, 56: 8652–8656
Tao HB, Xu Y, Huang X, Chen J, Pei L, Zhang J, Chen JG, Liu B. Joule, 2019, 3: 1498–1509
Huang ZF, Song J, Du Y, Xi S, Dou S, Nsanzimana JMV, Wang C, Xu ZJ, Wang X. Nat Energy, 2019, 4: 329–338
Dincă M, Surendranath Y, Nocera DG. Proc Natl Acad Sci USA, 2010, 107: 10337–10341
Bai L, Lee S, Hu X. Angew Chem Int Ed, 2021, 60: 3095–3103
Giordano L, Han B, Risch M, Hong WT, Rao RR, Stoerzinger KA, Shao-Horn Y. Catal Today, 2016, 262: 2–10
Moonshiram D, Purohit V, Concepcion JJ, Meyer TJ, Pushkar Y. Materials, 2013, 6: 392–409
Pasquini C, Zaharieva I, González-Flores D, Chernev P, Mohammadi MR, Guidoni L, Smith RDL, Dau H. J Am Chem Soc, 2019, 141: 2938–2948
Krishtalik LI. BioChim Biophysica Acta (BBA) — Bioenergetics, 2000, 1458: 6–27
Young ER, Rosenthal J, Hodgkiss JM, Nocera DG. J Am Chem Soc, 2009, 131: 7678–7684
Hammes-Schiffer S. J Am Chem Soc, 2015, 137: 8860–8871
Brammer L, Bruton EA, Sherwood P. Cryst Growth Des, 2001, 1: 277–290
Chaudhari SR, Mogurampelly S, Suryaprakash N. J Phys Chem B, 2013, 117: 1123–1129
Chen Z, Vannucci AK, Concepcion JJ, Jurss JW, Meyer TJ. Proc Natl Acad Sci USA, 2011, 108: E1461–E1469
Acknowledgements
This work was conducted in the Fundamental Research Center of Artificial Photosynthesis (FReCAP), and financially supported by the National Natural Science Foundation of China (22172011 and 22088102), the K&A Wallenberg Foundation (KAW 2016.0072), and Key Laboratory of Bio-based Chemicals of Liaoning Province of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2021_1178_MOESM1_ESM.docx
Promotion of the Oxygen Evolution Catalytic Performance of Ni-Fe Layered Hydroxides via the Introduction of a Proton Transfer Mediator Anion, approximately 6.47 MB.
Rights and permissions
About this article
Cite this article
Li, W., Li, F., Zhao, Y. et al. Promotion of the oxygen evolution performance of Ni-Fe layered hydroxides via the introduction of a proton-transfer mediator anion. Sci. China Chem. 65, 382–390 (2022). https://doi.org/10.1007/s11426-021-1178-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1178-y