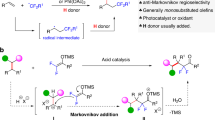
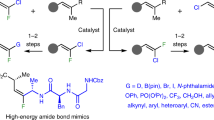
Abstract
The steric-switched ipso-defluoroamination-triggered and ipso-defluorooxylation-triggered cyclization of (trifluoromethyl)alkenes with amino alcohols and diamines are achieved under mild conditions. This regioselective strategy distinguishes the different nucleophilic heteroatom sites in amino alcohols and unsymmetric diamines by the sequential defluorinative functionalization of two C(sp3)-F bonds in a CF3 group. Various attractive monofluoroalkene-masked medium-sized heterocyclic lactams and lactones are obtained in moderate to excellent yields. Simple derivation of these masked-heterocycles efficiently affords useful skeletons of lactams, lactones, and 1,4-oxazepanes in a single diastereoisomer. Mechanism studies indicate that a unique sequential ipso-/γ-selective defluorinative functionalization pathway is involved in these transformations.

Similar content being viewed by others
References
Baud LG, Manning MA, Arkless HL, Stephens TC, Unsworth WP. Chem Eur J, 2017, 23: 2225–2230
Lawer A, Rossi-Ashton JA, Stephens TC, Challis BJ, Epton RG, Lynam JM, Unsworth WP. Angew Chem Int Ed, 2019, 58: 13942–13947
Zhu BH, Zheng YX, Kang W, Deng C, Zhou JM, Ye LW. Sci China Chem, 2021, 64: 1985–1989
Shiina I. Chem Rev, 2007, 107: 239–273
Uno H, Kawai K, Shiro M, Shibata N. ACS Catal, 2020, 10: 14117–14126
Wohlfahrt M, Harms K, Koert U. Angew Chem Int Ed, 2011, 50: 8404–8406
Maslivetc VA, Turner DN, McNair KN, Frolova L, Rogelj S, Maslivetc AA, Aksenov NA, Rubina M, Rubin M. J Org Chem, 2018, 83: 5650–5664
Wattanasin S, Albert R, Ehrhardt C, Roche D, Sabio M, Hommel U, Welzenbach K, Weitz-Schmidt G. Bioorg Med Chem Lett, 2003, 13: 499–502
Smits G, Zemribo R. Eur J Org Chem, 2015, 2015(14): 3152–3156
Smits G, Zemribo R. Org Biomol Chem, 2020, 18: 4566–4568
Purser S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev, 2008, 37: 320–330
Hagmann WK. J Med Chem, 2008, 51: 4359–4369
Furuya T, Kamlet AS, Ritter T. Nature, 2011, 473: 470–477
Li C, Liao Y, Tan X, Liu X, Liu P, Lv WX, Wang H. Sci China Chem, 2021, 64: 999–1003
Landelle G, Bergeron M, Turcotte-Savard MO, Paquin JF. Chem Soc Rev, 2011, 40: 2867–2908
Yanai H, Taguchi T. Eur J Org Chem, 2011, 2011(30): 5939–5954
Yang J, Mao A, Yue Z, Zhu W, Luo X, Zhu C, Xiao Y, Zhang J. Chem Commun, 2015, 51: 8326–8329
Chelucci G. Chem Rev, 2012, 112: 1344–1462
Zhang X, Cao S. Tetrahedron Lett, 2017, 58: 375–392
Jaroschik F. Chem Eur J, 2018, 24: 14572–14582
Lemal DM. J Org Chem, 2004, 69: 1–11
O’Hagan D. Chem Soc Rev, 2008, 37: 308–319
Burdeniuc J, Jedicka B, Crabtree RH. Chem Ber Recl, 1997, 130: 145–154
Papaianina O, Amsharov KY. Chem Commun, 2016, 52: 1505–1508
Yamada T, Saito K, Akiyama T. Adv Synth Catal, 2016, 358: 62–66
Fujita T, Fuchibe K, Ichikawa J. Angew Chem Int Ed, 2019, 58: 390–402
Tian F, Yan G, Yu J. Chem Commun, 2019, 55: 13486–13505
Wang M, Pu X, Zhao Y, Wang P, Li Z, Zhu C, Shi Z. J Am Chem Soc, 2018, 140: 9061–9065
Jang YJ, Rose D, Mirabi B, Lautens M. Angew Chem Int Ed, 2018, 57: 16147–16151
Yan SS, Wu DS, Ye JH, Gong L, Zeng X, Ran CK, Gui YY, Li J, Yu DG. ACS Catal, 2019, 9: 6987–6992
Xu W, Jiang H, Leng J, Ong HW, Wu J. Angew Chem Int Ed, 2020, 59: 4009–4016
Yue WJ, Day CS, Martin R. J Am Chem Soc, 2021, 143: 6395–6400
Zhu C, Sun MM, Chen K, Liu H, Feng C. Angew Chem Int Ed, 2021, 60: 20237–20242
Fuchibe K, Takahashi M, Ichikawa J. Angew Chem Int Ed, 2012, 51: 12059–12062
Ichitsuka T, Fujita T, Arita T, Ichikawa J. Angew Chem Int Ed, 2014, 53: 7564–7568
Zeng H, Cai Y, Jiang H, Zhu C. Org Lett, 2021, 23: 66–70
Yu YJ, Zhang FL, Peng TY, Wang CL, Cheng J, Chen C, Houk KN, Wang YF. Science, 2021, 371: 1232–1240
Zeng H, Zhu C, Jiang H. Org Lett, 2019, 21: 1130–1133
Cai Y, Zeng H, Zhu C, Liu C, Liu G, Jiang H. Org Chem Front, 2020, 7: 1260–1265
Zeng H, Zhu C, Liu C, Cai Y, Chen F, Jiang H. Chem Commun, 2020, 56: 6241–6244
Zhu C, Zeng H, Liu C, Cai Y, Fang X, Jiang H. Org Lett, 2020, 22: 809–813
CCDC 2081847 (3fa), 2102762 (3im), 2081846 (4na), 2102761 (6ga), and 2081850 (9nm) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
Trost BM, Li CJ. Modern Alkyne Chemistry. Catalytic and Atom-Economic Transformations. Weinheim: Wiley-VCH, 2015
Shaw S, Bian Z, Zhao B, Tarr JC, Veerasamy N, Jeon KO, Belmar J, Arnold AL, Fogarty SA, Perry E, Sensintaffar JL, Camper DMV, Rossanese OW, Lee T, Olejniczak ET, Fesik SW. J Med Chem, 2018, 61: 2410–2421
Lee T, Christov PP, Shaw S, Tarr JC, Zhao B, Veerasamy N, Jeon KO, Mills JJ, Bian Z, Sensintaffar JL, Arnold AL, Fogarty SA, Perry E, Ramsey HE, Cook RS, Hollingshead M, Davis Millin M, Lee KM, Koss B, Budhraja A, Opferman JT, Kim K, Arteaga CL, Moore WJ, Olejniczak ET, Savona MR, Fesik SW. J Med Chem, 2019, 62: 3971–3988
Chen L, Liu Y, Song H, Liu Y, Wang L, Wang Q. Mol Divers, 2017, 21: 61–68
Alvarez SG, Botyanszki J, De Los Angeles J, Fu J, Fujimoto R, Gralapp JM, Griffith RC, Lu P, Pham SM, Roberts CD, Schmitz FU, Seepersaud M, Tommasi R, Villa AC, Wattanasin S, Yifru A, Zheng H, Zheng X. PCT Int Appl. 2009, WO 20090816129 A1
Audouze K, Nielsen EØ, Peters D. J Med Chem, 2004, 47: 3089–3104
Bergeron M, Guyader D, Paquin JF. Org Lett, 2012, 14: 5888–5891
Fujita T, Morioka R, Arita T, Ichikawa J. Chem Commun, 2018, 54: 12938–12941
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21702064) and the Guangdong Basic and Applied Basic Research Foundation (2020B1515020012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Zeng, H., Li, H., Jiang, H. et al. Steric-switched defluorofunctionalization selectivity: controlled synthesis of monofluoroalkene-masked medium-sized heterocyclic lactams and lactones. Sci. China Chem. 65, 554–562 (2022). https://doi.org/10.1007/s11426-021-1135-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1135-8