Abstract
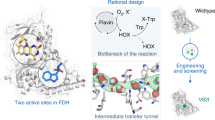
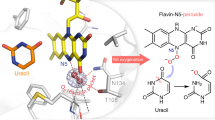
Flavin-dependent halogenases (FDHs) are well known to introduce carbon halide bonds (mainly C-Cl and C-Br) into natural products with the assistance of a partner protein flavin reductase to generate reduced flavin (FADH2 or FMNH2). Compared with the common chloride- and bromide-containing natural products (approximately 5,000 compounds), iodinated natural products (approximately 100 compounds) are very limited. Specific iodinases have also rarely been identified in nature to date. This study discovered a novel relationship between iodination and flavin reductases for the first time. Through mechanistic studies, it was identified that peroxide (H2O2) was released from the uncoupling reaction of flavin reductases and then reacted with iodide ions (I−) to produce hypoiodous acid (IOH) for the final iodination. Furthermore, this study also unintentionally verified that the recently reported flavin-dependent iodinase VirX1 from the marine virus and its two homologs (MBG and NCV) did not catalyze iodination in the in vitro biochemical system but likely belonged to a new phylogenetic clade in the tryptophan halogenase superfamily. As a consequence, actual flavin-dependent iodinases in nature remain to be discovered by the scientific community in the future.

Similar content being viewed by others
References
Neumann CS, Fujimori DG, Walsh CT. Chem Biol, 2008, 15: 99–109
Xu Z, Yang Z, Liu Y, Lu Y, Chen K, Zhu W. J Chem Inf Model, 2014, 54: 69–78
Jeschke P. Pest Manag Sci, 2010, 66: 10–27
Ludewig H, Molyneux S, Ferrinho S, Guo K, Lynch R, Gkotsi DS, Goss RJ. Curr Opin Struct Biol, 2020, 65: 51–60
Felpin FX, Sengupta S. Chem Soc Rev, 2019, 48: 1150–1193
Ruiz-Castillo P, Buchwald SL. Chem Rev, 2016, 116: 12564–12649
Schmidt R, Stolle A, Ondruschka B. Green Chem, 2012, 14: 1673–1679
Prakash GKS, Mathew T, Hoole D, Esteves PM, Wang Q, Rasul G, Olah GA. J Am Chem Soc, 2004, 126: 15770–15776
Gkotsi DS, Dhaliwal J, McLachlan MM, Mulholand KR, Goss RJ. Curr Opin Chem Biol, 2018, 43: 119–126
Yeh E, Garneau S, Walsh CT. Proc Natl Acad Sci USA, 2005, 102: 3960–3965
Eustáquio AS, Pojer F, Noel JP, Moore BS. Nat Chem Biol, 2008, 4: 69–74
Mitchell AJ, Zhu Q, Maggiolo AO, Ananth NR, Hillwig ML, Liu X, Boal AK. Nat Chem Biol, 2016, 12: 636–640
Furtmüller PG, Zederbauer M, Jantschko W, Helm J, Bogner M, Jakopitsch C, Obinger C. Archives Biochem Biophys, 2006, 445: 199–213
Carvalho DP, Dupuy C. Mol Cellular Endocrinol, 2017, 458: 6–15
Gkotsi DS, Ludewig H, Sharma SV, Connolly JA, Dhaliwal J, Wang Y, Unsworth WP, Taylor RJK, McLachlan MMW, Shanahan S, Naismith JH, Goss RJM. Nat Chem, 2019, 11: 1091–1097
Schnepel C, Turner NJ. Nat Chem, 2019, 11: 1076–1078
van Pée KH, Ligon JM. Nat Prod Rep, 2000, 17: 157–164
Dong C, Flecks S, Unversucht S, Haupt C, van Pée KH, Naismith JH. Science, 2005, 309: 2216–2219
Payne JT, Andorfer MC, Lewis JC. Angew Chem Int Ed, 2013, 52: 5271–5274
Tiwari MK, Singh RK, Lee JK, Zhao H. Bioorg Medicinal Chem Lett, 2012, 22: 1344–1347
Lee JK, Zhao H. J Bacteriol, 2007, 189: 8556–8563
Ingelman M, Ramaswamy S, Nivière V, Fontecave M, Eklund H. Biochemistry, 1999, 38: 7040–7049
Shepherd SA, Menon BRK, Fisk H, Struck AW, Levy C, Leys D, Micklefield J. ChemBioChem, 2016, 17: 821–824
Sun K, Lv Y, Wang J, Sun J, Liu L, Jia M, Liu X, Li Z, Wang X. Org Lett, 2015, 17: 4408–4411
Holtmann D, Hollmann F. ChemBioChem, 2016, 17: 1391–1398
Blasiak LC, Drennan CL. Acc Chem Res, 2009, 42: 147–155
Neubauer PR, Widmann C, Wibberg D, Schröder L, Frese M, Kottke T, Kalinowski J, Niemann HH, Sewald N. PLoS ONE, 2018, 13: e0196797
Ismail M, Frese M, Patschkowski T, Ortseifen V, Niehaus K, Sewald N. Adv Synth Catal, 2019, 361: 2475–2486
Widmann C, Ismail M, Sewald N, Niemann HH. Acta Crystlogr D Struct Biol, 2020, 76: 687–697
Acknowledgements
The authors thank Mrs. B. Dai and J. Wu at the Instrumental Analysis Center of Shanghai Jiao Tong University for making NMR experiments. This work was supported by the National Natural Science Foundation of China (21632007, 21661140002 for S. Lin; 81903525 for Y. Zhang), Research Fund for High-level Talents of Xinxiang Medical University (300-505272), and Open Funding Project of State Key Laboratory of Microbial Metabolism, Shanghai Jiao Tong University (MMLKF20-11).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Rights and permissions
About this article
Cite this article
Zhang, Y., Chen, L., Chen, H. et al. Aryl C-H iodination: are there actual flavin-dependent iodinases in nature?. Sci. China Chem. 64, 1730–1735 (2021). https://doi.org/10.1007/s11426-021-1018-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1018-0