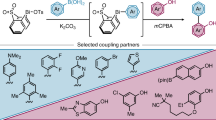
Abstract
Privileged biaryl frameworks, BINOL and NOBIN, were efficiently constructed with sole 1-DNQs as arylation reagents under one set of reaction conditions. The judicious selection of palladium-catalytic system plays a pivotal role in the excellent selectivities. This transformation accommodated fairly broad substrate generality for both 2-naphthol and N-Boc-2-naphthylamine and afforded the structurally diversified BINOLs and NOBIN derivatives in high efficiency. Notably, the bromo-substituents which cannot survive in conventional palladium-catalyzed reactions were well-compatible with this set of conditions, providing an effective handle for further enriching the library of BINOLs and NOBINs. Preliminary attempts on the asymmetric variant of this reaction were also performed with up to 80:20 er for BINOLs synthesis.

Similar content being viewed by others
References
Pu L. Chem Rev, 1998, 98: 2405–2494
Chen Y, Yekta S, Yudin AK. Chem Rev, 2003, 103: 3155–3212
Kočovský P, Vyskočil Š, Smrčina M. Chem Rev, 2003, 103: 3213–3246
Brunel JM. Chem Rev, 2005, 105: 857–898
Ding K, Li X, Ji B, Guo H, Kitamura M. COS, 2005, 2: 499–545
Ding K, Guo H, Li X, Yuan Y, Wang Y. Top Catal, 2005, 35: 105–116
Bringmann G, Gulder T, Gulder TAM, Breuning M. Chem Rev, 2011, 111: 563–639
McCormick MH, McGuire JM, Pittenger GE, Pittenger RC, Stark WM. Antibiot Annu, 1955–1956, 3: 606–611
Bremner JB, Keller PA, Pyne SG, Boyle TP, Brkic Z, David DM, Robertson M, Somphol K, Baylis D, Coates JA, Deadman J, Jeevarajah D, Rhodes DI. BioOrg Medicinal Chem, 2010, 18: 2611–2620
Pu L. Acc Chem Res, 2012, 45: 150–163
Takaishi K, Yasui M, Ema T. J Am Chem Soc, 2018, 140: 5334–5338
Cui Y, Lee SJ, Lin W. J Am Chem Soc, 2003, 125: 6014–6015
For selected examples, see: (a) Dewar MJS, Nakaya T. J Am Chem Soc, 1968, 90: 7134–7135
Hovorka M, Günterová J, Závada J. Tetrahedron Lett, 1990, 31: 413–416
Li X, Yang J, Kozlowski MC. Org Lett, 2001, 3: 1137–1140
Guo QX, Wu ZJ, Luo ZB, Liu QZ, Ye JL, Luo SW, Cun LF, Gong LZ. J Am Chem Soc, 2007, 129: 13927–13938
Egami H, Katsuki T. J Am Chem Soc, 2009, 131: 6082–6083
Narute S, Parnes R, Toste FD, Pappo D. J Am Chem Soc, 2016, 138: 16553–16560
Tian J, Wang A, Yang J, Zhao X, Tu Y, Zhang S, Chen Z. Angew Chem Int Ed, 2019, 58: 11023–11027
Shen D, Xu Y, Shi SL. J Am Chem Soc, 2019, 141: 14938–14945
Hayashi H, Ueno T, Kim C, Uchida T. Org Lett, 2020, 22: 1469–1474
Qiu H, Shuai B, Wang YZ, Liu D, Chen YG, Gao PS, Ma HX, Chen S, Mei TS. J Am Chem Soc, 2020, 142: 9872–9878
Chen YH, Cheng DJ, Zhang J, Wang Y, Liu XY, Tan B. J Am Chem Soc, 2015, 137: 15062–15065
Moliterno M, Cari R, Puglisi A, Antenucci A, Sperandio C, Moretti E, Di Sabato A, Salvio R, Bella M. Angew Chem Int Ed, 2016, 55: 6525–6529
Coombs G, Sak MH, Miller SJ. Angew Chem Int Ed, 2020, 59: 2875–2880
Wang JZ, Zhou J, Xu C, Sun H, Kürti L, Xu QL. J Am Chem Soc, 2016, 138: 5202–5205
Chen YH, Qi LW, Fang F, Tan B. Angew Chem Int Ed, 2017, 56: 16308–16312
Zhang J, Qi L, Li S, Xiang S, Tan B. Chin J Chem, 2020, 38: 1503–1514
Zhang SS, Jiang CY, Wu JQ, Liu XG, Li Q, Huang ZS, Li D, Wang H. Chem Commun, 2015, 51: 10240–10243
Jia ZJ, Merten C, Gontla R, Daniliuc CG, Antonchick AP, Waldmann H. Angew Chem Int Ed, 2017, 56: 2429–2434
Liu Z, Wu JQ, Yang SD. Org Lett, 2017, 19: 5434–5437
Somai Magar KB, Edison TNJI, Lee YR. Eur J Org Chem, 2017, 47: 7046–7054
Wu K, Cao B, Zhou CY, Che CM. Chem Eur J, 2018, 24: 4815–4819
Jang YS, Woźniak Ł, Pedroni J, Cramer N. Angew Chem Int Ed, 2018, 57: 12901–12905
Takahashi S, Shimooka H, Okauchi T, Kitamura M. Chem Lett, 2019, 48: 28–31
Wu K, Wu L, Zhou C, Che C. Angew Chem Int Ed, 2020, 59: 16202–16208
Wang H, Richard Y, Wan Q, Zhou C, Che C. Angew Chem Int Ed, 2020, 59: 1845–1850
Wang YB, Tan B. Acc Chem Res, 2018, 51: 534–547
Qi LW, Li S, Xiang SH, Wang JJ, Tan B. Nat Catal, 2019, 2: 314–323
Ding WY, Yu P, An QJ, Bay KL, Xiang SH, Li S, Chen Y, Houk KN, Tan B. Chem, 2020, 6: 2046–2059
Yan S, Xia W, Li S, Song Q, Xiang SH, Tan B. J Am Chem Soc, 2020, 142: 7322–7327
For reviews, see: (a) Miller DJ, Moody CJ. Tetrahedron, 1995, 51: 10811–10843; For selected examples, see
Yates P. J Am Chem Soc, 1952, 74: 5376–5381
Maier TC, Fu GC. J Am Chem Soc, 2006, 128: 4594–4595
Chen C, Zhu SF, Liu B, Wang LX, Zhou QL. J Am Chem Soc, 2007, 129: 12616–12617
Xie XL, Zhu SF, Guo JX, Cai Y, Zhou QL. Angew Chem Int Ed, 2014, 53: 2978–2981
Yu Z, Ma B, Chen M, Wu HH, Liu L, Zhang J. J Am Chem Soc, 2014, 136: 6904–6907
Yu Z, Li Y, Zhang P, Liu L, Zhang J. Chem Sci, 2019, 10: 6553–6559
Ma B, Tang Z, Zhang J, Liu L. Chem Commun, 2020, 56: 9485–9488
Li X, Wang J, Xie X, Dai W, Han X, Chen K, Liu H. Chem Commun, 2020, 56: 3441–3444
Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew Chem Int Ed, 2004, 43: 1566–1568
Uraguchi D, Terada M. J Am Chem Soc, 2004, 126: 5356–5357
Volla CMR, Atodiresei I, Rueping M. Chem Rev, 2014, 114: 2390–2431
Zhu SF, Zhou QL. Acc Chem Res, 2012, 45: 1365–1377
Guo X, Hu W. Acc Chem Res, 2013, 46: 2427–2440
Xia Y, Qiu D, Wang J. Chem Rev, 2017, 117: 13810–13889
Xiao Q, Zhang Y, Wang J. Acc Chem Res, 2013, 46: 236–247
Liu Y, Yu Z, Zhang JZ, Liu L, Xia F, Zhang J. Chem Sci, 2016, 7: 1988–1995
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21825105), the Guangdong Provincial Key Laboratory of Catalysis (2020B121201002), the Guangdong Innovative Program (2019BT02Y335), the Shenzhen Nobel Prize Scientists Laboratory Project (C17213101), and the SUSTech Special Fund for the Construction of High-Level Universities (G02216402). The authors appreciate the assistance of SUSTech Core Research Facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, JW., Jiang, F., Chen, YH. et al. Synthesis of structurally diversified BINOLs and NOBINs via palladium-catalyzed C-H arylation with diazoquinones. Sci. China Chem. 64, 1515–1521 (2021). https://doi.org/10.1007/s11426-021-1003-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1003-9