Abstract
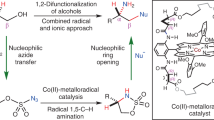
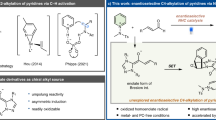
Chiral β-amino alcohols are important building blocks for the synthesis of drugs, natural products, chiral auxiliaries, chiral ligands and chiral organocatalysts. The catalytic asymmetric β-amination of alcohols offers a direct strategy to access this class of molecules. Herein, we report a general intramolecular C(sp3)-H nitrene insertion method for the synthesis of chiral oxazolidin-2-ones as precursors of chiral β-amino alcohols. Specifically, the ring-closing C(sp3)-H amination of N-benzoyloxycarbamates with 2 mol% of a chiral ruthenium catalyst provides cyclic carbamates in up to 99% yield and with up to 99% ee. The method is applicable to benzylic, allylic, and propargylic C-H bonds and can even be applied to completely non-activated C (sp3)-H bonds, although with somewhat reduced yields and stereoselectivities. The obtained cyclic carbamates can subsequently be hydrolyzed to obtain chiral β-amino alcohols. The method is very practical as the catalyst can be easily synthesized on a gram scale and can be recycled after the reaction for further use. The synthetic value of the new method is demonstrated with the asymmetric synthesis of a chiral oxazolidin-2-one as intermediate for the synthesis of the natural product aurantioclavine and chiral β-amino alcohols that are intermediates for the synthesis of chiral amino acids, indane-derived chiral Box-ligands, and the natural products dihydrohamacanthin A and dragmacidin A.

Similar content being viewed by others
References
Bergmeier SC. Tetrahedron, 2000, 56: 2561–2576
Heravi MM, Lashaki TB, Fattahi B, Zadsirjan V. RSC Adv, 2018, 8: 6634–6659
Ager DJ, Prakash I, Schaad DR. Chem Rev, 1996, 96: 835–876
Fache F, Schulz E, Tommasino ML, Lemaire M. Chem Rev, 2000, 100: 2159–2232
Robak MAT, Herbage MA, Ellman JA. Chem Rev, 2010, 110: 36003740
Donohoe TJ, Callens CKA, Flores A, Lacy AR, Rathi AH. Chem Eur J, 2011, 17: 58–76
Li G, Chang HT, Sharpless KB. Angew Chem Int Ed Engl, 1996, 35: 451–454
Nilov D, Reiser O. Adv Synthesis Catal, 2002, 344: 1169–1173
Pavlidis IV, Weiβ MS, Genz M, Spurr P, Hanlon SP, Wirz B, Iding H, Bornscheuer UT. Nat Chem, 2016, 8: 1076–1082
Gupta P, Mahajan N. New J Chem, 2018, 42: 12296–12327
Nakafuku KM, Zhang Z, Wappes EA, Stateman LM, Chen AD, Nagib DA. Nat Chem, 2020, 12: 697–704
Che CM, Lo VKY, Zhou CY, Huang JS. Chem Soc Rev, 2011, 40: 1950
Dequirez G, Pons V, Dauban P. Angew Chem Int Ed, 2012, 51: 7384–7395
Roizen JL, Harvey ME, Du Bois J. Acc Chem Res, 2012, 45: 911–922
Park Y, Kim Y, Chang S. Chem Rev, 2017, 117: 9247–9301
Hayashi H, Uchida T. Eur J Org Chem, 2020, 2020: 909–916
van Vliet KM, de Bruin B. ACS Catal, 2020, 10: 4751–4769
Liang JL, Yuan SX, Huang JS, Yu WY, Che CM. Angew Chem Int Ed, 2002, 41: 3465–3468
Milczek E, Boudet N, Blakey S. Angew Chem Int Ed, 2008, 47: 6825–6828
Zalatan DN, Du Bois J. J Am Chem Soc, 2008, 130: 9220–9221
Lebel H, Huard K, Lectard S. J Am Chem Soc, 2005, 127: 14198–14199
Huard K, Lebel H. Chem Eur J, 2008, 14: 6222–6230
Reddy RP, Davies HML. Org Lett, 2006, 8: 5013–5016
Zheng Y, Tan Y, Harms K, Marsch M, Riedel R, Zhang L, Meggers E. J Am Chem Soc, 2017, 139: 4322–4325
For ruthenium catalyzed asymmetric C(sp3)-H aminations through transition metal nitrenoids, see for example: (a) Zhou XG, Yu XQ, Huang JS, Che CM. Chem Commun, 1999, 2377–2378
Nishioka Y, Uchida T, Katsuki T. Angew Chem Int Ed, 2013, 52: 1739–1742
Xing Q, Chan CM, Yeung YW, Yu WY. J Am Chem Soc, 2019, 141: 3849–3853
Zhou Z, Chen S, Hong Y, Winterling E, Tan Y, Hemming M, Harms K, Houk KN, Meggers E. J Am Chem Soc, 2019, 141: 19048–19057
Li L, Han F, Nie X, Hong Y, Ivlev S, Meggers E. Angew Chem Int Ed, 2020, 59: 12392–12395
For recent contribtions from other groups regarding asymmetric catalysis with chiral-at-metal complexes, see for example: (a) Carmona M, Rodríguez R, Passarelli V, Lahoz FJ, García-Orduña P, Carmona D. J Am Chem Soc, 2018, 140: 912–915
Liang H, Xu GQ, Feng ZT, Wang ZY, Xu PF. J Org Chem, 2019, 84: 60–72
Qurban S, Du Y, Gong J, Lin SX, Kang Q. Chem Commun, 2019, 55: 249–252
Wan Q, Li S, Kang Q, Yuan Y, Du Y. J Org Chem, 2019, 84: 15201–15211
Hu L, Lin S, Li S, Kang Q, Du Y. ChemCatChem, 2020, 12: 118–121
Qin J, Zhou Z, Cui T, Hemming M, Meggers E. Chem Sci, 2019, 10: 3202–3207
Zhou Z, Chen S, Qin J, Nie X, Zheng X, Harms K, Riedel R, Houk KN, Meggers E. Angew Chem Int Ed, 2019, 58: 1088–1093
Zhou Z, Tan Y, Yamahira T, Ivlev S, Xie X, Riedel R, Hemming M, Kimura M, Meggers E. Chem, 2020, 6: 2024–2034
Prosser TJ, Marcantonio AF, Genge CA, Breslow DS. Tetrahedron Lett, 1964, 5: 2483–2487
Jiang H, Lang K, Lu H, Wojtas L, Zhang XP. J Am Chem Soc, 2017, 139: 9164–9167
Lee J, Lee J, Jung H, Kim D, Park J, Chang S. J Am Chem Soc, 2020, 142: 12324–12332
Tan Y, Chen S, Zhou Z, Hong Y, Ivlev S, Houk KN, Meggers E. Angew Chem Int Ed, 2020, 59: 21706–21710
Tan Y, Han F, Hemming M, Wang J, Harms K, Xie X, Meggers E. Org Lett, 2020, 22: 6653–6656
The method also works for the amination of C(sp2)-H bonds, see Supporting Information online for more details
For a recent example of a ruthenium-catalyzed asymmetric C(sp2)-H functionalization, see: Li G, Liu Q, Vasamsetty L, Guo W, Wang J. Angew Chem Int Ed, 2020, 59: 3475–3479
Skepper CK, Moreau RJ, Appleton BA, Benton BM, Drumm III JE, Feng BY, Geng M, Hu C, Li C, Lingel A, Lu Y, Mamo M, Mergo W, Mostafavi M, Rath CM, Steffek M, Takeoka KT, Uehara K, Wang L, Wei JR, Xie L, Xu W, Zhang Q, de Vicente J. J Med Chem, 2018, 61: 3325–3349
Ermert P, Meyer J, Stucki C, Schneebeli J, Obrecht JP. Tetrahedron Lett, 1988, 29: 1265–1268
Davies IW, Senanayake CH, Larsen RD, Verhoeven TR, Reider PJ. Tetrahedron Lett, 1996, 37: 813–814
Desimoni G, Faita G, Jergensen KA. Chem Rev, 2006, 106: 3561–3651
Park J, Kim DH, Das T, Cho CG. Org Lett, 2016, 18: 5098–5101
Yang CG, Wang J, Tang XX, Jiang B. Tetrahedron-Asymmetry, 2002, 13: 383–394
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (ME 1805/15-1). We thank Marcel Hemming for the synthesis of some substrate intermediates.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zhou, Z., Tan, Y., Shen, X. et al. Catalytic enantioselective synthesis of β-amino alcohols by nitrene insertion. Sci. China Chem. 64, 452–458 (2021). https://doi.org/10.1007/s11426-020-9906-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-020-9906-x