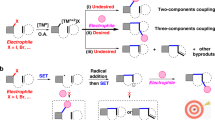
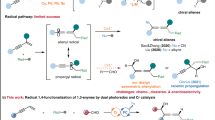
Abstract
A photoredox/palladium-cocatalyzed enantioselective alkylation of racemic secondary carbonates with 4-alkyl-1,4-dihydropyridines under visible light irradiation has been developed. The present study provides a method for the preparation of optically active diarylalkanes from racemic diarylmethyl carbonates by a dynamic kinetic asymmetric transformation (DYKAT). This photoredox/palladium dual catalysis strategy expands the scope of the asymmetric Pd-catalyzed benzylic substitution reaction and serves as its potential alternative and complement.
Similar content being viewed by others
References
Tsuji J, Minami I. Acc Chem Res, 1987, 20: 140–145
Trost BM, Crawley ML. Chem Rev, 2003, 103: 2921–2944
Trost BM, Machacek MR, Aponick A. Acc Chem Res, 2006, 39: 747–760
Trost BM. Tetrahedron, 2015, 71: 5708–5733
Tsuji J. Tetrahedron, 2015, 71: 6330–6348
Trost BM, Dietsch TJ. J Am Chem Soc, 1973, 95: 8200–8201
Trost BM, Strege PE. J Am Chem Soc, 1977, 99: 1649–1651
Trost B, Schultz J. Synthesis, 2019, 51: 1–30
For reviews on benzylic alkylations via π-benzylpalladium intermediates, see:(a)_Trost BM, Czabaniuk LC. Angew Chem Int Ed, 2014, 53: 2826–2851
Le Bras J, Muzart J. Eur J Org Chem, 2016, 15: 2565–2593
Trost BM, Czabaniuk LC. J Am Chem Soc, 2010, 132: 15534–15536
Trost BM, Czabaniuk LC. J Am Chem Soc, 2012, 134: 5778–5781
Schwarz KJ, Yang C, Fyfe JWB, Snaddon TN. Angew Chem Int Ed, 2018, 57: 12102–1210105
Faber K. Chem Eur J, 2001, 7: 5004–5010
Trost BM, Fandrick MR. Aldrichim Acta, 2007, 40: 59–72
Steinreiber J, Faber K, Griengl H. Chem Eur J, 2008, 14: 8060–8072
Bhat V, Welin ER, Guo X, Stoltz BM. Chem Rev, 2017, 117: 4528–4561
Wencel-Delord J, Colobert F. Synthesis, 2016, 48: 2981–2996
Legros JY, Toffano M, Fiaud JC. Tetrahedron, 1995, 51: 3235–3246
Legros JY, Boutros A, Fiaud JC, Toffano M. J Mol Catal A-Chem, 2003, 196: 21–25
Tabuchi S, Hirano K, Miura M. Angew Chem Int Ed, 2016, 55: 6973–6977
Najib A, Hirano K, Miura M. Org Lett, 2017, 19: 2438–2441
Matsude A, Hirano K, Miura M. Org Lett, 2018, 20: 3553–3556
Najib A, Hirano K, Miura M. Chem Eur J, 2018, 24: 6525–6529
For selected examples on benzylic substitution in a racemic manner, see: (a) Kuwano R, Kondo Y, Matsuyama Y. J Am Chem Soc, 2003, 125: 12104–12105
Kuwano R, Kondo Y. Org Lett, 2004, 6: 3545–3547
Kuwano R, Kusano H. Chem Lett, 2007, 36: 528–529
Torregrosa RRP, Ariyarathna Y, Chattopadhyay K, Tunge JA. J Am Chem Soc, 2010, 132: 9280–9282
Yasuda S, Ishii T, Takemoto S, Haruki H, Ohmiya H. Angew Chem Int Ed, 2018, 57: 2938–2942
For selected examples on Ni-catalyzed benzylic substitutions, see: (a) Taylor BLH, Swift EC, Waetzig JD, Jarvo ER. J Am Chem Soc, 2011, 133: 389–391
Taylor BLH, Harris MR, Jarvo ER. Angew Chem Int Ed, 2012, 51: 7790–7793
Greene MA, Yonova IM, Williams FJ, Jarvo ER. Org Lett, 2012, 14: 4293–4296
Harris MR, Hanna LE, Greene MA, Moore CE, Jarvo ER. J Am Chem Soc, 2013, 135: 3303–3306
Wisniewska HM, Swift EC, Jarvo ER. J Am Chem Soc, 2013, 135: 9083–9090
Yonova IM, Johnson AG, Osborne CA, Moore CE, Morrissette NS, Jarvo ER. Angew Chem Int Ed, 2014, 53: 2422–2427
Martin-Montero R, Krolikowski T, Zarate C, Manzano R, Martin R. Synlett, 2017, 28: 2604–2608
Zhou Q, Srinivas HD, Dasgupta S, Watson MP. J Am Chem Soc, 2013, 135: 3307–3310
Arp FO, Fu GC. J Am Chem Soc, 2005, 127: 10482–10483
Binder JT, Cordier CJ, Fu GC. J Am Chem Soc, 2012, 134: 17003–17006
Do HQ, Chandrashekar ERR, Fu GC. J Am Chem Soc, 2013, 135: 16288–16291
Anthony D, Lin Q, Baudet J, Diao T. Angew Chem Int Ed, 2019, 58: 3198–3202
For selected examples using “hard” nucleophiles, see: (a) Trost BM, Thaisrivongs DA, Hartwig J. J Am Chem Soc, 2011, 133: 12439–12441
Ardolino MJ, Morken JP. J Am Chem Soc, 2014, 136: 7092–7100
Misale A, Niyomchon S, Luparia M, Maulide N. Angew Chem Int Ed, 2014, 53: 7068–7073
Mao J, Zhang J, Jiang H, Bellomo A, Zhang M, Gao Z, Dreher SD, Walsh PJ. Angew Chem Int Ed, 2016, 55: 2526–2530
Murakami R, Sano K, Iwai T, Taniguchi T, Monde K, Sawamura M. Angew Chem Int Ed, 2018, 57: 9465–9469
Li TR, Maliszewski ML, Xiao WJ, Tunge JA. Org Lett, 2018, 20: 1730–1734
Mendis SN, Tunge JA. Org Lett, 2015, 17: 5164–5167
Zhou Z, Nie X, Harms K, Riedel R, Zhang L, Meggers E. Sci China Chem, 2019, 62: 1512–1518
For selected reviews on the merge of photoredox and transtion metal catalysis, see: (a) Skubi KL, Blum TR, Yoon TP. Chem Rev, 2016, 116: 10035–10074
Tellis JC, Kelly CB, Primer DN, Jouffroy M, Patel NR, Molander GA. Acc Chem Res, 2016, 49: 1429–1439
Twilton J, Le C, Zhang P, Shaw MH, Evans RW, MacMillan DWC. Nat Rev Chem, 2017, 1: 0052
Parasram M, Gevorgyan V. Chem Soc Rev, 2017, 46: 6227–6240
Prier CK, Rankic DA, MacMillan DWC. Chem Rev, 2013, 113: 5322–5363
Chen Y, Lu LQ, Yu DG, Zhu CJ, Xiao WJ. Sci China Chem, 2019, 62: 24–57
For a review on transition metals and photoredox cocatalyzed allylic substitutions, see: (a) Zhang HH, Yu S. Acta Chim Sin, 2019, 77: 832–840
Matsui JK, Gutiérrez-Bonet Á, Rotella M, Alam R, Gutierrez O, Molander GA. Angew Chem Int Ed, 2018, 57: 15847–15851
Thullen SM, Rovis T. J Am Chem Soc, 2017, 139: 15504–15508
Zheng J, Breit B. Angew Chem Int Ed, 2019, 58: 3392–3397
Schwarz JL, Schäfers F, Tlahuext-Aca A, Lückemeier L, Glorius F. J Am Chem Soc, 2018, 140: 12705–12709
Mitsunuma H, Tanabe S, Fuse H, Ohkubo K, Kanai M. Chem Sci, 2019, 10: 3459–3465
Chen M, Zhao MN, Zhang YD, Ren ZH, Guan ZH. Sci China Chem, 2018, 61: 695–701
Noble A, Aggarwal VK. Sci China Chem, 2019, 62: 1083–1084
Lang SB, O’Nele KM, Tunge JA. J Am Chem Soc, 2014, 136: 13606–13609
Lang SB, O’Nele KM, Douglas JT, Tunge JA. Chem Eur J, 2015, 21: 18589–18593
Xuan J, Zeng TT, Feng ZJ, Deng QH, Chen JR, Lu LQ, Xiao WJ, Alper H. Angew Chem Int Ed, 2015, 54: 1625–1628
Zhang HH, Zhao JJ, Yu S. J Am Chem Soc, 2018, 140: 16914–16919
Gutiérrez-Bonet Á, Remeur C, Matsui JK, Molander GA. J Am Chem Soc, 2017, 139: 12251–12258
Zhang HH, Yu S. J Org Chem, 2017, 82: 9995–10006
Verrier C, Alandini N, Pezzetta C, Moliterno M, Buzzetti L, Hepburn HB, Vega-Peñaloza A, Silvi M, Melchiorre P. ACS Catal, 2018, 8: 1062–1066
Huang W, Cheng X. Synlett, 2017, 28: 148–158
Kalyani D, McMurtrey KB, Neufeldt SR, Sanford MS. J Am Chem Soc, 2011, 133: 18566–18569
Neufeldt SR, Sanford MS. Adv Synth Catal, 2012, 354: 3517–3522
Zhou C, Li P, Zhu X, Wang L. Org Lett, 2015, 17: 6198–6201
Sharma UK, Gemoets HPL, Schröder F, Noël T, Van der Eycken EV. ACS Catal, 2017, 7: 3818–3823
Powers DC, Ritter T. Nat Chem, 2009, 1: 302–309
Powers DC, Benitez D, Tkatchouk E, Goddard III WA, Ritter T. J Am Chem Soc, 2010, 132: 14092–14103
Maestri G, Malacria M, Derat E. Chem Commun, 2013, 49: 10424–10426
Choi S, Chatterjee T, Choi WJ, You Y, Cho EJ. ACS Catal, 2015, 5: 4796–4802
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21971110, 21732003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2019_9732_MOESM1_ESM.pdf
Photoredox/palladium-cocatalyzed enantioselective alkylation of secondary benzyl carbonates with 4-alkyl-1,4-dihydropyridines
Rights and permissions
About this article
Cite this article
Shen, X., Qian, L. & Yu, S. Photoredox/palladium-cocatalyzed enantioselective alkylation of secondary benzyl carbonates with 4-alkyl-1,4-dihydropyridines. Sci. China Chem. 63, 687–691 (2020). https://doi.org/10.1007/s11426-019-9732-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-019-9732-5