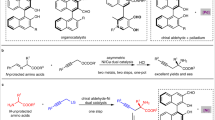
Abstract
A catalytic enantioselective three-component Ugi reaction was developed. SPINOL-derived phosphoric acid with bulky 2,4,6-tricyclohexylphenyl groups at the 6,6′ positions was found to be the best catalyst to afford α-amino amide derivatives in good to excellent yields (62% to 99%) and enantiocontrol (81% to >99% enantiomeric excess). This asymmetric reaction was applicable well to an array of aliphatic aldehydes. The gram-scale synthesis, modification of dapsone, and enantioselective synthesis of (R)-Lacosamide underline the general utility of this methodology Influence of dihedral angles and substituents of the chiral phosphoric acids on the enantioselectivity was also discussed in this article.
Similar content being viewed by others
References
Kent SBH. Chem Soc Rev, 2009, 38: 338–351
D’Hondt M, Bracke N, Taevernier L, Gevaert B, Verbeke F, Wynendaele E, De Spiegeleer B. J Pharm Biomed Anal, 2014, 101: 2–30
Wan R, Bai R, Yan C, Lei J, Shi Y. Cell, 2019, 177: 339–351
Welsch ME, Kaplan A, Chambers JM, Stokes ME, Bos PH, Zask A, Zhang Y, Sanchez-Martin M, Badgley MA, Huang CS, Tran TH, Akkiraju H, Brown LM, Nandakumar R, Cremers S, Yang WS, Tong L, Olive KP, Ferrando A, Stockwell BR. Cell, 2017, 168: 878–889
Mons E, Jansen IDC, Loboda J, van Doodewaerd BR, Hermans J, Verdoes M, van Boeckel CAA, van Veelen PA, Turk B, Turk D, Ovaa H. J Am Chem Soc, 2019, 141: 3507–3514
Miley GP, Rote JC, Silverman RB, Kelleher NL, Thomson RJ. Org Lett, 2018, 20: 2369–2373
McGrath NA, Brichacek M, Njardarson JT. J Chem Educ, 2010, 87: 1348–1349
Pelagatti P, Carcelli M, Calbiani F, Cassi C, Elviri L, Pelizzi C, Rizzotti U, Rogolino D. Organometallics, 2005, 24: 5836–5844
Burguete MI, Collado M, Escorihuela J, Luis SV. Angew Chem Int Ed, 2007, 46: 9002–9005
Yu Z, Liu X, Zhou L, Lin L, Feng X. Angew Chem Int Ed, 2009, 48: 5195–5198
Banik SM, Levina A, Hyde AM, Jacobsen EN. Science, 2017, 358: 761–764
Altava B, Burguete MI, Carbó N, Escorihuela J, Luis SV. Tetrahedron-Asymmetry, 2010, 21: 982–989
Gorla L, Martí-Centelles V, Altava B, Burguete MI, Luis SV. Dalton Trans, 2017, 46: 2660–2669
Zhang W, Liu F, Zhang C, Luo JG, Luo J, Yu W, Kong L. Anal Chem, 2017, 89: 12319–12326
Katritzky AR, Mohapatra P. P, Singh S, Clemens N, Kirichenko K. J Serb Chem Soc, 2005, 70: 319–327
Valeur E, Bradley M. Chem Soc Rev, 2009, 38: 606–631
Pattabiraman VR, Bode JW. Nature, 2011, 480: 471–479
Zhu YP, Mampuys P, Sergeyev S, Ballet S, Maes BUW. Adv Synth Catal, 2017, 359: 2481–2498
Ugi I, Dömling A, Hörl W. Endeavour, 1994, 18: 115–122
Dömling A, Ugi I. Angew Chem Int Ed, 2000, 39: 3168–3210
Ruijter E, Scheffelaar R, Orru RVA. Angew Chem Int Ed, 2011, 50: 6234–6246
Dömling A. Chem Rev, 2006, 106: 17–89
Ugi I, Steinbrückner C. Angew Chem, 1960, 72: 267–268
Ugi I, Meyr R, Fetzer U, Steinbrückner C. Angew Chem, 1959, 71: 386
Pan SC, List B. Angew Chem Int Ed, 2008, 47: 3622–3625
Tomlin FM, Gerling-Driessen UIM, Liu YC, Flynn RA, Vangala JR, Lentz CS, Clauder-Muenster S, Jakob P, Mueller WF, Ordoñez-Rueda D, Paulsen M, Matsui N, Foley D, Rafalko A, Suzuki T, Bogyo M, Steinmetz LM, Radhakrishnan SK, Bertozzi CR. ACS Cent Sci, 2017, 3: 1143–1155
Noyori R. Angew Chem Int Ed, 2002, 41: 2008–2022
Xiao KJ, Lin DW, Miura M, Zhu RY, Gong W, Wasa M, Yu JQ. J Am Chem Soc, 2014, 136: 8138–8142
Wang Q, Wang DX, Wang MX, Zhu J. Acc Chem Res, 2018, 51: 1290–1300
Luo W, Yuan X, Lin L, Zhou P, Liu X, Feng X. Chem Sci, 2016, 7: 4736–4740
Xiong Q, Dong S, Chen Y, Liu X, Feng X. Nat Commun, 2019, 10: 2116
Yue T, Wang MX, Wang DX, Masson G, Zhu J. Angew Chem Int Ed, 2009, 48: 6717–6721
Su Y, Bouma MJ, Alcaraz L, Stocks M, Furber M, Masson G, Zhu J. Chem Eur J, 2012, 18: 12624–12627
Hashimoto T, Kimura H, Kawamata Y, Maruoka K. Angew Chem Int Ed, 2012, 51: 7279–7281
Zhang Y, Ao YF, Huang ZT, Wang DX, Wang MX, Zhu J. Angew Chem Int Ed, 2016, 55: 5282–5285
Zhao W, Huang L, Guan Y, Wulff WD. Angew Chem Int Ed, 2014, 53: 3436–3441
Zhang J, Yu P, Li SY, Sun H, Xiang SH, Wang JJ, Houk KN, Tan B. Science, 2018, 361: eaas8707
Zhang J, Lin SX, Cheng DJ, Liu XY, Tan B. J Am Chem Soc, 2015, 137: 14039–14042
Xu JH, Zheng SC, Zhang JW, Liu XY, Tan B. Angew Chem Int Ed, 2016, 55: 11834–11839
Zhang LL, Zhang JW, Xiang SH, Guo Z, Tan B. Chin J Chem, 2018, 36: 1182–1186
Shaabani A, Keshipour S, Shaabani S, Mahyari M. Tetrahedron Lett, 2012, 53: 1641–1644
Zhang J, Shi W, Liu Q, Chen T, Zhou X, Yang C, Zhang K, Xie Z. Polym Chem, 2018, 9: 5566–5571
Akiyama T. Chem Rev, 2007, 107: 5744–5758
Parmar D, Sugiono E, Raja S, Rueping M. Chem Rev, 2014, 114: 9047–9153
Reid JP, Goodman JM. Chem Eur J, 2017, 23: 14248–14260
Zhu YI, Stiller MJ. J Am Acad Dermatol, 2001, 45: 420–434
Beyreuther BK, Freitag J, Heers C, Krebsfänger N, Scharfenecker U, Stöhr T. CNS Drug Rev, 2007, 13: 21–42
Wehlan H, Oehme J, Schäfer A, Rossen K. Org Process Res Dev, 2015, 19: 1980–1986
Xie Y, Zhao Y, Qian B, Yang L, Xia C, Huang H. Angew Chem Int Ed, 2011, 50: 5682–5686
Xu B, Zhu SF, Zhang ZC, Yu ZX, Ma Y, Zhou QL. Chem Sci, 2014, 5: 1442–1448
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21825105, 21772081), Shenzhen Special Funds for the Development of Biomedicine, Internet, New Energy, and New Material Industries (JCYJ20170412151701379, KQJSCX20170328153203), Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (PDJH2019C467).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, YY., Sun, H. et al. Enantioselective three-component Ugi reaction catalyzed by chiral phosphoric acid. Sci. China Chem. 63, 47–54 (2020). https://doi.org/10.1007/s11426-019-9606-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-019-9606-2