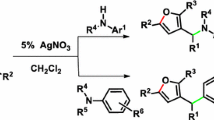
Abstract
A novel Ag(I)-catalyzed benzylic amination reaction with in situ generation of NH-1,2,3-triazoles for N2-substituted 1,2,3-triazole scaffolds is described. This protocol is achieved with easily accessible substrate, broad functional group, good regioselectivity, thus providing the efficient and practical method to diverse N2-substituted 1,2,3-triazole rings with moderate to good yields.
Similar content being viewed by others
References
Davies HML, Alford JS. Chem Soc Rev, 2014, 43: 5151–5162
Huang D, Zhao P, Astruc D. Coordin Chem Rev, 2014, 272: 145–165
Hosseyni S, Wojtas L, Li M, Shi X. J Am Chem Soc, 2016, 138: 3994–3997
Ye X, Xu C, Wojtas L, Akhmedov NG, Chen H, Shi X. Org Lett, 2016, 18: 2970–2973
Goitia A, Gómez-Bengoa E, Correa A. Org Lett, 2017, 19: 962–965
Kacprzak K, Skiera I, Piasecka M, Paryzek Z. Chem Rev, 2016, 116: 5689–5743
Kolb HC, Sharpless KB. Drug Discovery Today, 2003, 8: 1128–1137
Whiting M, Tripp JC, Lin YC, Lindstrom W, Olson AJ, Elder JH, Sharpless KB, Fokin VV. J Med Chem, 2006, 49: 7697–7710
Wilkinson BL, Bornaghi LF, Houston TA, Poulsen SA. In: Kaplan S P, ed. Drug Design Research Perspectives. Nova: Haupauge, 2007. 57
Chrovian CC, Soyode-Johnson A, Peterson AA, Gelin CF, Deng X, Dvorak CA, Carruthers NI, Lord B, Fraser I, Aluisio L, Coe KJ, Scott B, Koudriakova T, Schoetens F, Sepassi K, Gallacher DJ, Bhattacharya A, Letavic MA. J Med Chem, 2018, 61: 207–223
Zheng Y, Ji X, Yu B, Ji K, Gallo D, Csizmadia E, Zhu M, Choudhury MR, De La Cruz LKC, Chittavong V, Pan Z, Yuan Z, Otterbein LE, Wang B. Nat Chem, 2018, 10: 787–794
Tiwari VK, Mishra BB, Mishra KB, Mishra N, Singh AS, Chen X. Chem Rev, 2016, 116: 3086–3240
Kim WG, Choi B, Yang HJ, Han JA, Jung H, Cho HJ, Kang S, Hong SY. Bioconjugate Chem, 2016, 27: 2007–2013
Thirumurugan P, Matosiuk D, Jozwiak K. Chem Rev, 2013, 113: 4905–4979
Tang W, Becker ML. Chem Soc Rev, 2014, 43: 7013–7039
Tian H, Fürstenberg A, Huber T. Chem Rev, 2017, 117: 186–245
Finn MG, Fokin VV. Chem Soc Rev, 2010, 39: 1231
Kumar AS, Ghule VD, Subrahmanyam S, Sahoo AK. Chem Eur J, 2013, 19: 509–518
Hawker CJ, Fokin VV, Finn MG, Sharpless KB. Aust J Chem, 2007, 60: 381–383
Chu C, Liu R. Chem Soc Rev, 2011, 40: 2177–2188
Astruc D, Liang L, Rapakousiou A, Ruiz J. Acc Chem Res, 2012, 45: 630–640
Leophairatana P, Samanta S, De Silva CC, Koberstein JT. J Am Chem Soc, 2017, 139: 3756–3766
Huisgen R. Angew Chem Int Ed Engl, 1963, 2: 565–598
Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed, 2001, 40: 2004–2021
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed, 2002, 41: 2596–2599
Tornøe CW, Christensen C, Meldal M. J Org Chem, 2002, 67: 3057–3064
Fokin VV. In: Ding K, ai L-X, eds. Organic Chemistry-Breakthroughs and Perspectives. Weinheim: Wiley-VCH, 2012. 247–277
Banert K. In: Bräse S, Banert K, Eds. Organic Azides: Syntheses and Applications. Chichester: Wiley, 2010
Wei F, Wang W, Ma Y, Tung CH, Xu Z. Chem Commun, 2016, 52: 14188–14199
Kappe CO, Van der Eycken E. Chem Soc Rev, 2010, 39: 1280–1290
Johansson JR, Beke-Somfai T, Said Stålsmeden A, Kann N. Chem Rev, 2016, 116: 14726–14768
Wang Z, Li B, Zhang X, Fan X. J Org Chem, 2016, 81: 6357–6363
Wang Q, Shi X, Zhang X, Fan X. Org Biomol Chem, 2017, 15: 8529–8534
Kim WG, Kang ME, Lee JB, Jeon MH, Lee S, Lee J, Choi B, Cal PMSD, Kang S, Kee JM, Bernardes GJL, Rohde JU, Choe W, Hong SY. J Am Chem Soc, 2017, 139: 12121–12124
Zhu L, Zhang H, Wang C, Chen Z. Chin J Org Chem, 2018, 38: 1052–1064
Chen Y, Liu Y, Petersen JL, Shi X. Chem Commun, 2008, 2: 3254–3256
Kalisiak J, Sharpless KB, Fokin VV. Org Lett, 2008, 10: 3171–3174
Wang XJ, Sidhu K, Zhang L, Campbell S, Haddad N, Reeves DC, Krishnamurthy D, Senanayake CH. Org Lett, 2009, 11: 5490–5493
Wang X, Zhang L, Krishnamurthy D, Senanayake CH, Wipf P. Org Lett, 2010, 12: 4632–4635
Yan W, Wang Q, Chen Y, Petersen JL, Shi X. Org Lett, 2010, 12: 3308–3311
Liu Y, Yan W, Chen Y, Petersen JL, Shi X. Org Lett, 2008, 10: 5389–5392
Zhang Y, Ye X, Petersen JL, Li M, Shi X. J Org Chem, 2015, 80: 3664–3669
Wang X, Zhang L, Lee H, Haddad N, Krishnamurthy D, Senanayake CH. Org Lett, 2009, 11: 5026–5028
Ueda S, Su M, Buchwald SL. Angew Chem Int Ed, 2011, 50: 8944–8947
Ueda S, Ali S, Fors BP, Buchwald SL. J Org Chem, 2012, 77: 2543–2547
Guru MM, Punniyamurthy T. J Org Chem, 2012, 77: 5063–5073
Stewart S, Harris R, Jamieson C. Synlett, 2014, 25: 2480–2484
Wu L, Guo S, Wang X, Guo Z, Yao G, Lin Q, Wu M. Tetrahedron Lett, 2015, 56: 2145–2148
Gavlik KD, Lesogorova SG, Sukhorukova ES, Subbotina JO, Slepukhin PA, Benassi E, Belskaya NP. Eur J Org Chem, 2016, 2016: 2700–2710
Zhou J, He J, Wang B, Yang W, Ren H. J Am Chem Soc, 2011, 133: 6868–6870
Ryu T, Min J, Choi W, Jeon WH, Lee PH. Org Lett, 2014, 16: 2810–2813
Li J, Zhou H, Zhang J, Yang H, Jiang G. Chem Commun, 2016, 52: 9589–9592
Kamijo S, Jin T, Huo Z, Yamamoto Y. J Org Chem, 2003, 69: 2386–2393
Kalisiak J, Sharpless KB, Fokin VV. Org Lett, 2008, 10: 3171–3174
Peshkov VA, Pereshivko OP, Nechaev AA, Peshkov AA, Van der Eycken EV. Chem Soc Rev, 2018, 47: 3861–3898
Boyarskiy VP, Ryabukhin DS, Bokach NA, Vasilyev AV. Chem Rev, 2016, 116: 5894–5986
Yang L, Huang H. Chem Rev, 2015, 115: 3468–3517
Dorel R, Echavarren AM. Chem Rev, 2015, 115: 9028–9072
Asiri AM, Hashmi ASK. Chem Soc Rev, 2016, 45: 4471–4503
Zeng X. Chem Rev, 2013, 113: 6864–6900
Müller DS, Marek I. Chem Soc Rev, 2016, 45: 4552–4566
Fang G, Bi X. Chem Soc Rev, 2015, 44: 8124–8173
Sekine K, Yamada T. Chem Soc Rev, 2016, 45: 4524–4532
Ning Y, Ji Q, Liao P, Anderson EA, Bi X. Angew Chem Int Ed, 2017, 56: 13805–13808
Sadamitsu Y, Komatsuki K, Saito K, Yamada T. Org Lett, 2017, 19: 3191–3194
Wang H, Mi P, Zhao W, Kumar R, Bi X. Org Lett, 2017, 19: 5613–5616
Liu Z, Li Q, Liao P, Bi X. Chem Eur J, 2017, 23: 4756–4760
Barve IJ, Thikekar TU, Sun CM. Org Lett, 2017, 19: 2370–2373
Tang J, Sivaguru P, Ning Y, Zanoni G, Bi X. Org Lett, 2017, 19: 4026–4029
Liu JQ, Shen X, Wang Y, Wang XS, Bi X. Org Lett, 2018, 20: 6930–6933
Liu Z, Ji H, Gao W, Zhu G, Tong L, Lei F, Tang B. Chem Commun, 2017, 53: 6259–6262
Xu T, Hu X. Angew Chem Int Ed, 2015, 54: 1307–1311
Shen T, Wang T, Qin C, Jiao N. Angew Chem Int Ed, 2013, 52: 6677–6680
Meier BW, Gomez JD, Zhou A, Thompson JA. Chem Res Toxicol, 2005, 18: 1575–1585
Lemercier JN, Meier BW, Gomez JD, Thompson JA. Chem Res Toxicol, 2004, 17: 1675–1683
Kupfer R, Dwyer-Nield LD, Malkinson AM, Thompson JA. Chem Res Toxicol, 2002, 15: 1106–1112
Zhou Q, Turnbull KD. J Org Chem, 2001, 66: 7072–7077
(a) CCDC 1849952 of 3a-N1 (b) CCDC 1881654 of 3f-N2 (c) CCDC 1898986 of 3x (d) CCDC 1881653 of 4h (e) CCDC 1898987 of 4n. All the supplementary crystallographic data for this paper are from Cambridge Crystallographic Data Centre http://www.ccdc.cam.ac.uk/
Creary X, Anderson A, Brophy C, Crowell F, Funk Z. J Org Chem, 2012, 77: 8756–8761
Jin T, Kamijo S, Yamamoto Y. Eur J Org Chem, 2004, 2004: 3789–3791
Vitérisi A, Orsini A, Weibel JM, Pale P. Tetrahedron Lett, 2006, 47: 2779–2781
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21535004, 91753111, 21605097, 21775092, 21390411), the Key Research and Development Program of Shandong Province (2018YFJH0502), the Natural Science Foundation of Shandong Province of China (ZR2016BQ01, ZR2018JL008) and the China Postdoctoral Science Foundation (2017M610442).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, Z., Hao, W., Gao, W. et al. Silver-catalyzed three-component reaction: synthesis of N2-substituted 1,2,3-triazoles via direct benzylic amination. Sci. China Chem. 62, 1001–1006 (2019). https://doi.org/10.1007/s11426-019-9455-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-019-9455-0