Abstract
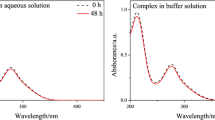
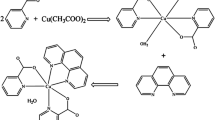
Dicyandiamide (DICY) is a common ligand that exhibits low toxicity but can irritate the skin and eyes and cause methemoglobinemia on long-term exposure. Crystalline Cu-dicyandiamide (Cu-DICY) was obtained via facile synthesis and its molecular structure and theoretical Raman spectra were simulated by using density functional theory (DFT). The results suggested that the Cu2+ coordinates with two H2O molecules and two different DICY molecules (an imino DICY and an amino DICY). The stability constants of Cu-DICY were calculated, and the electrochemical properties were studied. Two electro-chemical redox processes occur in Cu-DICY in an aqueous solution: a reversible reaction with a formal potential of 0.2 V vs. MSE and an irreversible reaction between –0.4 and –1.2 V vs. MSE. The standard rate constant k0 for the reversible reaction was estimated to be 7.6×10–3 cm/s. In addition, based on the reversible reaction of Cu-DICY, square wave voltammetry was used to rapidly determine the concentration of Cu(II) and the detection limit was 66.7 μg/L, which satisfies the detection limit requirements for copper in tap water (2 mg/L) as provided by the World Health Organization.
Similar content being viewed by others
References
Craymer L. The Wall Street Journal, 2013–1-25, ISSN: 0099–9660
Tskhovrebov AG, Bokach NA, Haukka M, Kukushkin VY. Inorg Chem, 2009, 48: 8678–8688
Williams PAM, Ferrer EG, Baeza N, Piro OE, Castellano EE, Baran EJ. Z anorg allg Chem, 2005, 631: 1502–1506
Ritche LK, Harrison WTA. Acta Crystlogr E Struct Rep Online, 2007, 63: m617–m618
Gad AAM. Z anorg allg Chem, 2012, 638: 1031–1038
Ma X, Li Y, Ye Z, Yang L, Zhou L, Wang L. J Hazard Mater, 2011, 185: 1348–1354
Kraft BJ, Eppley HJ, Huffman JC, Zaleski JM. J Am Chem Soc, 2002, 124: 272–280
Sunatsuki Y, Motoda Y, Matsumoto N. Coordin Chem Rev, 2002, 226: 199–209
Bailey PJ, Pace S. Coordin Chem Rev, 2001, 214: 91–141
Giorgetti M, Tonelli S, Zanelli A, Aquilanti G, Pellei M, Santini C. Polyhedron, 2012, 48: 174–180
Aquilanti G, Giorgetti M, Minicucci M, Papini G, Pellei M, Tegoni M, Trasatti A, Santini C. Dalton Trans, 2011, 40: 2764
Tian YQ, Luo HQ, Li NB. J Solid State Electrochem, 2012, 16: 529–533
Bobrowski A, Putek M, Zarębski J. Electroanalysis, 2012, 24: 1071–1078
Fei Y, Lv ZY, Wang AJ, Chen YH, Chen JR, Feng JJ. Microchim Acta, 2014, 181: 389–394
Liao Y, Li Q, Yue Y, Shao S. RSC Adv, 2015, 5: 3232–3238
Kerekovic I, Milardovic S, Palcic M, Grabaric Z. J Electroanal Chem, 2014, 724: 103–110
Kołodziej U, Przyłuski J. Polyhedron, 1985, 4: 395–399
Lu AJ, Lu CX, Wang Y. Chin J Spec Lab, 2006, 23: 506–508
Arbuznikov AV, Sheludyakova LA, Burgina EB. Chem Phys Lett, 1995, 240: 239–244
El-Nahas AM, Hirao K. J Phys Chem A, 2000, 104: 138–144
Nicholson RS. Anal Chem, 1965, 37: 1351–1355
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21275030, 21475023) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT_15R11). We also thank Prof. De-Yin Wu, Xiamen University for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xu, KX., Guo, MH., Ren, LQ. et al. Spectroscopic and electrochemical properties of Cu-dicyandiamide complex. Sci. China Chem. 61, 360–367 (2018). https://doi.org/10.1007/s11426-017-9178-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-017-9178-9