Abstract
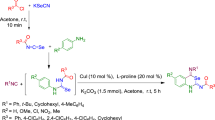
A new protocol has been developed for the formation of C−Se and C−S bonds by the direct selenosulfonylation of alkenes. This protocol is operationally simplistic, has a wide substrate scope, uses readily available seleno and sulfonyl sources, and is amenable to gram-scale synthesis. This reaction represents a significant addition to the limited number of reactions available for the intermolecular selenide difunctionalization of alkenes and would be useful for the synthesis of sulfur- and selenium-containing molecules
Similar content being viewed by others
References
Mugesh G, du Mont WW, Sies H. Chem Rev, 2001, 101: 2125–2180
Mugesh G, Singh HB. Acc Chem Res, 2002, 35: 226–236
Nogueira CW, Zeni G, Rocha JBT. Chem Rev, 2004, 104: 6255–6286
Rhoden CRB, Zeni G. Org Biomol Chem, 2011, 9: 1301–1313
Paulmier C. Selenium Reagents and Intermediates in Organic Synthesis. Organic Chemistry Series. Oxford, U.K.: Pergamon, 1986
Freudendahl DM, Santoro S, Shahzad SA, Santi C, Wirth T. Angew Chem Int Ed, 2009, 48: 8409–8411
Derek WJ, Risto L. Selenium and Tellurium Chemistry: from Small Molecules to Biomolecules and Materials. Berlin: Springer-Verlag, 2011
Zeni G, Larock RC. Chem Rev, 2004, 104: 2285–2310
Jensen KH, Sigman MS. Org Biomol Chem, 2008, 6: 4083–4088c
McDonald RI, Liu G, Stahl SS. Chem Rev, 2011, 111: 2981–3019
Cardona F, Goti A. Nat Chem, 2009, 1: 269–275
Wang Y, Zhang L, Yang Y, Zhang P, Du Z, Wang C. J Am Chem Soc, 2013, 135: 18048–18051
Zhou B, Ma P, Chen H, Wang C. Chem Commun, 2014, 50: 14558–14561
Ciesielski J, Dequirez G, Retailleau P, Gandon V, Dauban P. Chem Eur J, 2016, 22: 9338–9347
Courant T, Masson G. J Org Chem, 2016, 81: 6945–6952 and references therein
Ivachtchenko AV, Golovina ES, Kadieva MG, Kysil VM, Mitkin OD, Tkachenko SE, Okun IM. J Med Chem, 2011, 54: 8161–8173
Huang Y, Huo L, Zhang S, Guo X, Han CC, Li Y, Hou J. Chem Commun, 2011, 47: 8904–8906c
Kamigata N, Narushima T, Sawada H, Kobayashi M. Bull Chem Soc Jpn, 1984, 57: 1421–1422
Lu Q, Zhang J, Wei F, Qi Y, Wang H, Liu Z, Lei A. Angew Chem Int Ed, 2013, 52: 7156–7159
Huo C, Wang Y, Yuan Y, Chen F, Tang J. Chem Commun, 2016, 52: 7233–7236
Taniguchi N. J Org Chem, 2015, 80: 7797–7802
Wan X, Sun K, Zhang G. Sci China Chem, 2017, 60: doi: 10.1007/s11426-016-0284-2
Back TG, Collins S. J Org Chem, 1981, 46: 3249–3256
Black KA, Vogel P. J Org Chem, 1986, 51: 5341–5348
Gancarz RA, Kice JL. J Org Chem, 1981, 46: 4899–4906
Bäckvall JE, Nájera C, Yus M. Tetrahedron Lett, 1988, 29: 1445–1448
Sun K, Wang X, Lv Y, Li G, Jiao H, Dai C, Li Y, Zhang C, Liu L. Chem Commun, 2016, 52: 8471–8474
Sun K, Li Y, Xiong T, Zhang J, Zhang Q. J Am Chem Soc, 2011, 133: 1694–1697
Sun K, Wang X, Li G, Zhu Z, Jiang Y, Xiao B. Chem Commun, 2014, 50: 12880–12883
Sun K, Wang X, Liu L, Sun J, Liu X, Li Z, Zhang Z, Zhang G. ACS Catal, 2015, 5: 7194–7198
Sun K, Li Y, Zhang Q. Sci China Chem, 2015, 58: 1354–1358
Sun K, Lv Y, Wang J, Sun J, Liu L, Jia M, Liu X, Li Z, Wang X. Org Lett, 2015, 17: 4408–4411
Yanagisawa S, Ueda K, Taniguchi T, Itami K. Org Lett, 2008, 10: 4673–4676
Liu W, Cao H, Zhang H, Zhang H, Chung KH, He C, Wang H, Kwong FY, Lei A. J Am Chem Soc, 2010, 132: 16737–16740
Sun CL, Li H, Yu DG, Yu M, Zhou X, Lu XY, Huang K, Zheng SF, Li BJ, Shi ZJ. Nat Chem, 2010, 2: 1044–1049
Li X, Xu X, Hu P, Xiao X, Zhou C. J Org Chem, 2013, 78: 7343–7348
Shen T, Yuan Y, Song S, Jiao N. Chem Commun, 2014, 50: 4115
Wei W, Wen J, Yang D, Du J, You J, Wang H. Green Chem, 2014, 16: 2988–2991
Acknowledgments
This work was supported by the National Natural Science Foundation of China (U1504210), the China Postdoctoral Science Foundation (2015M572110), and Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis (130028651).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sun, K., Lv, Y., Shi, Z. et al. Direct access to β-seleno sulfones at room temperature through selenosulfonylation of alkenes. Sci. China Chem. 60, 730–733 (2017). https://doi.org/10.1007/s11426-016-0412-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-016-0412-0