Abstract
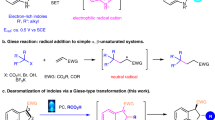
A convergent construction of [2,3]-fused indoline tetrahydropyridazines via an I2/tert-butyl hydroperoxide (TBHP) catalyzed three-component dearomative oxidative coupling of indoles, hydrazines and acetophenone was established in moderate to good yields. This protocol provides a new approach for the synthesis of these biologically interesting fused indolines.
Similar content being viewed by others
References
Haynes SW, Gao X, Tang Y, Walsh CT. ACS Chem Biol, 2013, 8: 741–748
Marcos IS, Moro RF, Costales I, Basabe P, Díez D. Nat Prod Rep, 2013, 30: 1509–1526
Kim J, Movassaghi M. Chem Soc Rev, 2009, 38: 3035–3050
Robertson FJ, Kenimer BD, Wu J. Tetrahedron, 2011, 67: 4327–4332
Yang J, Song H, Xiao X, Wang J, Qin Y. Org Lett, 2006, 8: 2187–2190
May JA, Stoltz B. Tetrahedron, 2006, 62: 5262–5271
May JA, Zeidan RK, Stoltz BM. Tetrahedron Lett, 2003, 44: 1203–1205
Zuo Z, Ma D. Angew Chem Int Ed, 2011, 50: 12008–12011
Zuo Z, Xie W, Ma D. J Am Chem Soc, 2010, 132: 13226–13228
Zi W, Zuo Z, Ma D. Acc Chem Res, 2015, 48: 702–711
Plescia S, Daidone G, Sprio V. J Heterocyclic Chem, 1979, 16: 805–806
Lopes SMM, Brigas AF, Palacios F, Lemos A, Pinhoe Melo TMVD. Eur J Org Chem, 2012, 2012: 2152–2160
Tong MC, Chen X, Li J, Huang R, Tao H, Wang CJ. Angew Chem Int Ed, 2014, 53: 4680–4684
Shao Z, Zhang H. Chem Soc Rev, 2012, 41: 560–572
Barluenga J, Valdés C. Angew Chem Int Ed, 2011, 50: 7486–7500
Xiao Q, Zhang Y, Wang J. Acc Chem Res, 2013, 46: 236–247
Chen Z, Yan Q, Yi H, Liu Z, Lei A, Zhang Y. Chem Eur J, 2014, 20: 13692–13697
Chen Z, Yan Q, Liu Z, Xu Y, Zhang Y. Angew Chem Int Ed, 2013, 52: 13324–13328
Cai ZJ, Lu XM, Zi Y, Yang C, Shen LJ, Li J, Wang SY, Ji SJ. Org Lett, 2014, 16: 5108–5111
Senadi GC, Hu WP, Lu TY, Garkhedkar AM, Vandavasi JK, Wang JJ. Org Lett, 2015, 17: 1521–1524
Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. J Am Chem Soc, 2008, 130: 17938–17954
Baran PS, Maimone TJ, Richter JM. Nature, 2007, 446: 404–408
Baran PS, Richter JM. J Am Chem Soc, 2005, 127: 15394–15396
Baran PS, Richter JM. J Am Chem Soc, 2004, 126: 7450–7451
Bienaymé H, Hulme C, Oddon G, Schmitt P. Chem Eur J, 2000, 6: 3321–3329
Posner GH. Chem Rev, 1986, 86: 831–844
Dömling A. Chem Rev, 2006, 106: 17–89
Wu XF, Gong JL, Qi X. Org Biomol Chem, 2014, 12: 5807–5817
Yan Y, Zhang Y, Feng C, Zha Z, Wang Z. Angew Chem Int Ed, 2012, 51: 8077–8081
Tang S, Liu K, Long Y, Gao X, Gao M, Lei A. Org Lett, 2015, 17: 2404–2407
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
11426_2016_170_MOESM1_ESM.pdf
Intermolecular Dearomative Oxidative Coupling of Indoles with Ketones and Sulfonylhydrazines Catalyzed by I2: Synthesis of [2,3]-Fused Indoline Tetrahydropyridazines
Rights and permissions
About this article
Cite this article
Wei, F., Cheng, L., Huang, H. et al. Intermolecular dearomative oxidative coupling of indoles with ketones and sulfonylhydrazines catalyzed by I2: synthesis of [2,3]-fused indoline tetrahydropyridazines. Sci. China Chem. 59, 1311–1316 (2016). https://doi.org/10.1007/s11426-016-0170-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-016-0170-8