Abstract
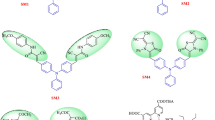
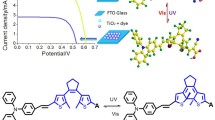
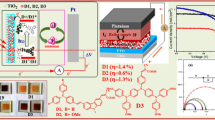
Two organic sensitizers (LI-33 and LI-34) with double anchoring units were synthesized and utilized for dye sensitized solar cells (DSSCs), which contained thiophene or vinyl thiophene as π-bridge. The introduction of double anchoring units can change their absorption spectra and energy levels in a large degree, thus, the better light-harvesting ability and the convenient electron transfer along the whole molecule can be obtained. The solar cell based on LI-34 exhibited a broad incident photon-to-current conversion efficiency (IPCE) spectrum and high conversion efficiency (η=6.05%) with coadsorbent CDCA.
Similar content being viewed by others
References
Armaroli N, Balzani V. The future of energy supply: challenges and opportunities. Angew Chem Int Ed, 2007, 46: 52–66
Listorti A, O’Regan B, Durrant JR. Electron transfer dynamics in dye-sensitized solar cells. Chem Mater, 2011, 23: 3381–3399
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H. Dye-sensitized solar cells. Chem Rev, 2010, 110: 6595–6663
O’Regan B, Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature, 1991, 353: 737–740
Ning Z, Tian, H. Triarylamine: a promising core unit for efficient photovoltaic materials. Chem Commun, 2009, 45: 5483–5495
Yum JH, Baranoff E, Wenger S, Nazeeruddin MK, Grätzel M. Panchromatic engineering for dye-sensitized solar cells. Energy Environ Sci, 2011, 4: 842–857
Calogero G, Di Marco G, Cazzanti S, Caramori S, Argazzi R, Bignozzi CA. Natural dye senstizers for photoelectrochemical cells Energy Environ Sci, 2009, 2: 1162–1172
Ooyama Y, Harima Y. Molecular designs and syntheses of organic dyes for dye-sensitized solar cells. Eur J Org Chem, 2009, 18: 2903–2934
Li Q, Lu L, Zhong C, Huang J, Huang Q, Shi J, Jin X, Peng T, Qin J, Li Z. New pyrrole-based organic dyes for dye-sensitized solar cells:convenient syntheses and high efficiency. Chem Eur J, 2009, 15: 9664–9668
Li Q, Lu L, Zhong C, Huang Q, Shi J, Jin X, Peng T, Qin J, Li Z. New indole-based metal-free organic dyes for dye-sensitized solar cells. J Phys Chem B, 2009, 113: 14588–14595
Liu B, Zhu W, Zhang Q, Wu W, Xu M, Ning Z, Xie Y, Tian H. Conveniently synthesized isophoronedyes for high efficiency dyesensitized solar cells: tuning photovoltaic performance by structural modification of donor group in donor-ΰ-acceptor system. Chem Commun, 2009, 45: 1766–1768
Shi J, Chai Z, Zhong C, Wu W, Hua J, Dong Y, Qin J, Li Q, Li Z. New efficient dyes containing tert-butyl in donor for dye-sensitized solar cells. Dyes Pigm, 2012, 95: 244–251
Li Q, Shi J, Li H, Li S, Zhong C, Guo F, Peng M, Hua J, Qin J, Li Z. Novel pyrrole-based dyes for dye-sensitized solar cells: from rod-shape to “H” type. J Mater Chem, 2012, 22: 6689–6696
Lu M, Liang M, Han H, Sun Z, Xue S. Organic dyes incorporating bis-hexapropyltruxeneamino moiety for efficient dye-sensitized solar cells. J Phys Chem C, 2011, 115: 274–281
Zhu W, Wu Y, Wang S, Li W, Li X, Chen J, Wang Z, Tian H. Organic D-A-ΰ-A solar cell sensitizers with improved stability and spectral response. Adv Funct Mater, 2011, 21: 756–763
Shi J, Huang J, Tang R, Chai Z, Hua J, Qin J, Li Q, Li Z. Efficient metal-free organic sensitizers containing tetraphenylethylene moieties in the donor part for dye-sensitized solar cells. Eur J Org Chem, 2012, 27: 5248–5255
Shi J, Chen J, Chai Z, Wang H, Tang R, Fan K, Wu M, Han H, Qin J, Peng T, Li Q, Li Z. High performance organic sensitizers based on 11,12-bis(hexyloxy) dibenzo[a,c]phenazine for dye-sensitized solar cells. J Mater Chem, 2012, 22: 18830–18838
Shi J, Chai Z, Su J, Chen J, Tang R, Fan K, Zhang L, Han H, Qin J, Peng T, Li Q, Li Z. New sensitizers bearing quinoxaline moieties as an auxiliary acceptor for dye-sensitized solar cells. Dyes Pigm, 2013, 98: 405–413
Ning Z, Fu Y, Tian H. Improvement of dye-sensitized solar cells: what we know and what we need to know. Energy Environ Sci, 2010, 3: 1170–1181
Abbotto A, Manfredi N, Marinzi C, De Angelis F, Mosconi E, Yum JH, Zhang X, Nazeeruddin MK, Grätzel M. Di-branched di-anchoring organic dyes for dye-sensitized solar cells. Energy Environ Sci, 2009, 2: 1094–1101
Shi L, He C, Zhu D, He Q, Li Y, Chen Y, Sun Y, Fu Y, Wen D, Cao H, Cheng J. High performance aniline vapor detection based on multi-branched fluorescent triphenylamine-benzothiadiazole derivatives: branch effect and aggregation control of the sensing performance. J Mater Chem, 2012, 22: 11629–11635
Goswami S, Manna A, Paul S, Aich K, Das AK, Chakraborty S. Highly reactive (<1 min) ratiometric probe for selective “naked-eye” detection of cyanide in aqueous media. Tetrahedron Lett, 2013, 54: 1785–1789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cros JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09. Wallingford CT: Gaussian, Inc., 2009
Koumura N, Wang Z, Mori S, Miyashita M, Suzuki E, Hara K. Alkyl-functionalized organic dyes for efficient molecular photovoltaics. J Am Chem Soc, 2006, 128: 14256–14257
Miyashita M, Sunahara K, Nishikawa T, Uemura YN, Hara K, Mori A, Abe T, Suzuki E, Mori S. Interfacial electron-transfer kinetics in metal-free organic dye-sensitized solar cells: combined effects of molecular structure of dyes and electrolytes. J Am Chem Soc, 2008, 130: 17874–17881
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shi, J., Chai, Z., Tang, R. et al. New triphenylamine-based sensitizers bearing double anchor units for dye-sensitized solar cells. Sci. China Chem. 58, 1144–1151 (2015). https://doi.org/10.1007/s11426-015-5411-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-5411-0