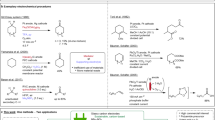
Abstract
Using NH4I as the supporting electrolyte as well as the precursor of an I2 promoter and nitrogen source, a highly efficient electrochemical route was developed to convert aldehydes to nitriles with excellent yields under mild reaction conditions. This electrochemical process could effectively avoid the direct use of NH3 gas, molecular iodine, and oxidants.
Similar content being viewed by others
References
Friedrich K, Waaensfesl K. The Chemistry of the Cyano Group. New York: Wiley-Interscience, 1970
Fatiadi AJ. Preparation and Synthetic Applications of Cyano Compounds. New York: Wiley, 1983
Chihiro M, Nagamoto H, Takemura I, Kitano K, Komatsu H, Sekiguchi K, Tabuse F, Mori T, Tominaga M, Yabuuchi Y. Novel thiazole derivatives as inhibitors of superoxide production by human neutrophils: synthesis and structure-activity relationships. J Med Chem, 1995, 38: 353–358
Serrano J, Sierra T, Gonzalez Y, Bolm C, Weickhardt K, Magnus A, Moli G. Improving FLC properties. Simplicity, planarity, and rigidity in new chiral oxazoline derivatives. J Am Chem Soc, 1995, 117: 8312–8321
Medwid JB, Paul R, Baker JS. Preparation of triazolo[1,5-c]pyrimidines as potential antiasthma agents. J Med Chem, 1990, 33: 1230–1241
Friedman L, Shechter H. Preparation of nitriles from halides and sodium cyanide. An advantageous nucleophilic displacement in dimethyl sulfoxide. J Org Chem, 1960, 25: 877–879
Bini L, Muller C, Wilting J, Chrzanowski L, Spek AL, Vogt D. Highly selective hydrocyanation of butadiene toward 3-pentenenitrile. J Am Chem Soc, 2007, 129: 12622–12623
Yang SH, Chang S. Highly efficient and catalytic conversion of aldoximes to nitriles. Org Lett, 2001, 3: 4209–4211
Harrison C, Hodge P, Rogers W. Conversion of carboxamides and oximes to nitriles or imidoyl chlorides using a polymer-supported phosphine and carbon tetrachloride. Synthesis, 1977, 1: 41–43
Cho BR, Chung HS, Cho NS. Elimination reactions of (E)-and (Z)-benzaldehyde o-benzoyloximes. Transition state differences for the syn- and anti-eliminations forming nitriles. J Org Chem, 1998, 63: 4685–4690
Maeyama K, Kobayashi M, Kato H, Yonezawa N. Nickel/zinc-media ted synthesis of aromatic nitriles from aromatic oxime ethers. Synthetic Commun, 2002, 32: 2519–2525
Stevens TE. The use of organodilithium compounds for the synthesis of 1,1-dimethylsilacyclopentane and 1,1-dimethylsilacyclohexane. J Org Chem, 1961, 26: 2530–2531
Iida S, Ohmura R, Togo H. Direct oxidative conversion of alkyl halides into nitriles with molecular iodine and 1,3-diiodo-5,5-dimethylhydantoin in aq ammonia. Tetrahedron, 2009, 65: 6257–6262
Leadbeater NE, Torenius HM, Tye H. Ionic liquids as reagents and solvents in conjunction with microwave heating: rapid synthesis of alkyl halides from alcohols and nitriles from aryl halides. Tetrahedron, 2003, 59: 2253–2258
Brackman W, Smit PJ. A new synthesis of nitriles. Recl Trav Chim, 1963, 82: 757–762
Misono A, Osa T, Koda S. The synthesis of nitriles from aldehydes. Bull Chem Soc Jpn, 1966, 39: 854
Erman MB, Snow JW, Williams MJ. A new efficient method for the conversion of aldehydes into nitriles using ammonia and hydrogen peroxide. Tetrahedron Lett, 2000, 41: 6749–6752
Carmeli M, Shefer N, Rozen S. From aldehydes to nitriles, a general and high yielding transformation using HOF·CH3CN complex. Tetrahedron Lett, 2006, 47: 8969–8972
Arote ND, Bhalerao DS, Akamanchi KG. Direct oxidative conversion of aldehydes to nitriles using IBX in aqueous ammonia. Tetrahedron Lett, 2007, 48: 3651–3653
Telvekar VN, Patel KN, Kundaikar HS. A novel system for the synthesis of nitriles from aldehydes using aqueous ammonia and sodium dichloroiodate. Tetrahedron Lett, 2008, 49: 2213–2215
Rajender Reddy K, Uma Maheswari C, Venkateshwar M. Catalytic oxidative conversion of alcohols, aldehydes and amines into nitriles using KI/I2-TBHP system. Tetrahedron Lett, 2009, 50: 2050–2053
Zhu C, Ji L, Wei Y. A facile and efficient one-pot synthesis of nitriles from aldehydes using hypervalent iodine (III) reagents in aqueous ammonium acetate. Synthesis, 2010, 18: 3121–3125
Zolfigol MA, Hajjami M, Ghorbani-Choghamarani A. A simple and one-pot oxidative conversion of alcohols or aldehydes to the nitriles using NaIO4/KI in aqueous NH3. Bull Korean Chem Soc, 2011, 32: 4191–4191
Bose DS, Narsaiah AV. Efficient one pot synthesis of nitriles from aldehydes in solid state using peroxymonosulfate on alumina. Tetrahedron Lett, 1998, 39: 6533–6534
Sridhar M, Reddy MKK, Sairam VV. Acetohydroxamic acid: a new reagent for efficient synthesis of nitriles directly from aldehydes using Bi(OTf)3 as the catalyst. Tetrahedron Lett, 2012, 53: 3421–3424
Enthaler S, Weidauer M, Schröder F. Straightforward zinc-catalyzed transformation of aldehydes and hydroxylamine hydrochloride to nitriles. Tetrahedron Lett, 2012, 53: 882–885
Talukdar S, Hsu JL, Chou TC. Direct transformation of aldehydes to nitriles using iodine in ammonia water. Tetrahedron Lett, 2001, 42: 1103–1105
Movassagh B, Shokri S. An efficient and convenient KF/Al2O3 mediated synthesis of nitriles from aldehydes. Tetrahedron Lett, 2005, 46: 6923–6925
Chill ST, Mebane RC. A facile one-pot conversion of aldehydes into nitriles. Synthetic Commun, 2009, 39: 3601–3606
Sharghi H, Sarvari MH. A direct synthesis of nitriles and amides from aldehydes using dry or wet alumina in solvent free conditions. Tetrahedron, 2002, 58: 10323–10328
Madhusudana Reddy MB, Pasha MA. Environment friendly protocol for the synthesis of nitriles from aldehydes. Chinese Chem Lett, 2010, 21: 1025–1028
Frontana-Uribe BA, Little RD, Ibanez JG, Palma A, Vasquez-Medrano R. Organic electrosynthesis: a promising green methodology in organic chemistry. Green Chem, 2010, 12: 2099–2119
Yoshida J, Kataoka K, Horcajada R, Nagaki A. Modern strategies in electroorganic synthesis. Chem Rev, 2008, 108: 2265–2299
Shono T, Matsumura Y, Tsubata K, Kamada T, Kishi K. Electrochemical transformation of aldoximes to nitriles using halogen ions as mediators: intermediary formation of nitrile oxides. J Org Chem, 1989, 54: 2249–2251
Francke R, Little RD. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem Soc Rev, 2014, 43: 2492–2521
Gao X, Yuan G, Chen H, Jiang H, Li Y, Qi C. Efficient conversion of CO2 with olefins into cyclic carbonates via a synergistic action of I2 and base electrochemically generated in situ. Electrochem Commun, 2013, 34: 242–245
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Qu, Q., Gao, X., Gao, J. et al. A highly efficient electrochemical route for the conversion of aldehydes to nitriles. Sci. China Chem. 58, 747–750 (2015). https://doi.org/10.1007/s11426-015-5331-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-5331-z