Abstract
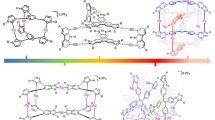
Catassembly is a new concept in molecular assembly that is analogous to catalysis in chemical synthesis. However, for most molecular-assembled processes, the catassembler contributions are rather inconspicuous due to the low activation barriers. As a result, few systems dealing with the catassembly are available until now. In this paper, we report that naphthalene diimide coordination networks are formed under the catassembly of lone-pair-bearing catassemblers (e.g., N,N-dimethylacetamide, N-methylpyrrolidin-2-one). During such molecular assembly, a stable transition state between the electron-deficient naphthalene diimide tectons and catassemblers via the less common lone pair-π interactions was observed, which is supposed to play the key role in the enhancement of coordination abilities of organic tectons and thus formation of the final coordination networks.
Similar content being viewed by others
References
Alivisatos P, Barbara PF, Castleman AW, Chang J, Dixon DA, Klein ML, McLendon GL, Miller JS, Ratner MA, Rossky PJ, Stupp SI, Thompson ME. Adv Mater, 1998, 10: 1297–1336
Lehn JM. Science, 2002, 295: 2400–2403
Philp D, Stoddart JF. Angew Chem Int Ed, 1996, 35: 1155–1196
Aida T, Meijer EW, Stupp SI. Science, 2012, 335: 813–817
Lee YS. Self-Assembly and Nanotechnology: A Force Balance Approach. Hoboken: Wiley, 2008
Noyori R. Asymmetric Catalysis in Organic Synthesis. New York: John Wiley, 1994. 16–94
List B. Chem Rev, 2007, 107: 5413–5415
MacMillan DWC. Nature, 2008, 455: 304–308
Dhakshinamoorthy A, Asiri AM, Garcia H. Chem Soc Rev, 2015, 44: 1922–1947
Wang Y, Lin HX, Ding SY, Liu DY, Chen L, Lei ZC, Fan FR, Tian ZQ. Sci China Chem, 2012, 42: 525–547
Wang Y, Lin HX, Chen L, Ding SY, Lei ZC, Liu DY, Cao XY, Liang HJ, Jiang YB, Tian ZQ. Chem Soc Rev, 2014, 43: 399–411
Prins J, De Jong F, Timmerman P, Reinhoudt DN. Nature, 2000, 408: 181–184
Lauceri R, Raudino A, Scolaro LM, Micali N, Purrello R. J Am Chem Soc, 2002, 124: 894–895
Zeng X, He YJ, Dai ZF, Wang J, Cao Q, Zhang YL. ChemPhysChem, 2009, 10: 954–962
Zhang L, Tian Y, MH Liu. Phys Chem Chem Phys, 2011, 13: 17205–17209
Qiu S, Xue M, Zhu G. Chem Soc Rev, 2014, 43: 6116–6140
Liu J, Chen L, Cui H, Zhang J, Zhang L, Su CY. Chem Soc Rev, 2014, 43: 6011–6061
Zhou HC, Long JR, Yaghi OM. Chem Rev, 2012, 112: 673–674
Stock N, Biswas S. Chem Rev, 2012, 112: 933–969
Murdock CR, Hughes BC, Lu Z, Jenkins DM. Coord Chem Rev, 2014, 258: 119–136
Zhao JP, Hu BW, Yang Q, Liu FC, Hu TL, Bu XH. Chin Sci Bull, 2009, 54: 2461–2466
Tian D, Liu S, Zhang D, Chang Z, Hu T, Bu X. Sci China Chem, 2013, 56: 1693–1700
Fujita M. Chem Soc Rev, 1998, 27: 417–425
Fox OD, Drew MGB, Beer PD. Angew Chem Int Ed, 2000, 39: 135–140
De S, Mahata K, Schmittel M. Chem Soc Rev, 2010, 39: 1555–1575
Brodin JD, Ambroggio XI, Tang C, Parent KN, Baker TS, Tezcan FA. Nat Chem, 2012, 4: 375–382
Guha S, Goodson FS, Corson LJ, Saha S. J Am Chem Soc, 2012, 134: 13679–13691
Liao JZ, Dui XJ, Zhang HL, Wu XY, Lu CZ. CrystEngComm, 2014, 16: 10530–10533
Fang X, Yuan X, Song YB, Wang JD, Lin MJ. CrystEngComm, 2014, 16: 9090–9095
Liu JJ, Wang Y, Hong YJ, Lin MJ, Huang CC, WX Dai. Dalton Trans, 2014, 43: 17908–17911
Liu JJ, Guan YF, Jiao C, Lin MJ, Huang CC, Dai WX. Dalton Trans, 2015, 44: 5957–5960
Liu JJ, Hong YJ, Guan YF, Lin MJ, Huang CC, Dai WX. Dalton Trans, 2015, 44: 653–658
You MH, Yuan X, Fang X, Lin MJ. Supramol Chem, 2015, 27: 460–464
Frontera A, Gamez P, Mascal M, Mooibroek TJ, Reedijk J. Angew Chem Int Ed, 2011, 50: 9564–9583
Gamez P. Inorg Chem Front, 2014, 1: 35–43
Bauzá A, Mooibroek TJ, Frontera A. Chem-PhysChem, 2015, 16: 2496–2517
Guha S, Saha S. J Am Chem Soc, 2010, 132: 17674–17677
Sheldrick GM. Acta Crystallogr, Sect A: Fundam Crystallogr, 2008, 64: 112–122
Spek AL. J Appl Crystallogr, 2003, 36: 7–13
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09. Revision D.01. Wallingford, CT: Gaussian Inc, 2013
Zahn S, Mac Farlane DR, Izgorodina EI. Phys Chem Chem Phys, 2013, 15: 13664–13675
Grimme S, Antony J, Ehrlich S, Krieg H. J Chem Phys, 2010, 132: 154104
Papajak E, Zheng J, Xu X, Leverentz HR, Truhlar DG. J Chem Theory Comput, 2011, 7: 3027–3034
Frisch MJ, Head-Gordon M, Pople JA. Chem Phys Lett, 1990, 166: 275–280
Boys SF, Bernardi F. Mol Phys, 1970, 19: 553–566
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W. J Am Chem Soc, 2010, 132: 6498–6506
Lu T, Chen F. J Comp Chem, 2012, 33: 580–592
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Liu, JJ., Fan, CR. et al. The catassembled generation of naphthalene diimide coordination networks with lone pair-π interactions. Sci. China Chem. 59, 1492–1497 (2016). https://doi.org/10.1007/s11426-015-0411-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-0411-2