Abstract
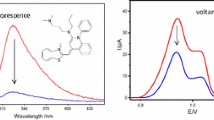
We have developed a simple method to synthesize 6-seleno-2′-deoxyguanosine (SedG) by selectively replacing the 6-oxygen atom with selenium. This selenium-atom-specific modification (SAM) alters the optical properties of the naturally occurring 2′-deoxyguanosine (dG). Unlike the native dG, the UVabsorption of SedG is significantly influenced by the pH of the aqueous solution. Moreover, SedG is fluorescent at the physiological pH and exhibits pH-dependent fluorescence in aqueous solutions. Furthermore, SedG has noticeable fluorescence in non-aqueous solutions, indicating its sensitivity to environmental changes. This is the first time a fluorescent nucleoside by single-atom alteration has been observed. Fluorescent nucleosides modified by a single atom have great potential as molecular probes with minimal perturbations to investigate nucleoside interactions with proteins, such as membrane-transporter proteins.
Similar content being viewed by others
References
Lin L, Sheng J, Huang Z. Nucleic acid X-ray crystallography via direct selenium derivatization. Chem Soc Rev, 2011, 40(9): 4591–4602
Salon J, Gan J, Abdur R, Liu H, Huang Z. Synthesis of 6-Seguanosine RNAs for structural study. Org Lett, 2013, (15)3934–3937
Sheng J, Gan J, Soars AS, Salon J, Huang Z. Structural insights of non-canonical U*U pair and hoogsteen interaction probed with Se atom. Nucleic Acids Res, 2013, in press
Sheng J, Zhang W, Hassan AE, Gan J, Soares AS, Geng S, Ren Y, Huang Z. Hydrogen bond formation between the naturally modified nucleobase and phosphate backbone. Nucleic Acids Res, 2012, 40(16): 8111–8118
Sheng J, Huang Z. Selenium derivatization of nucleic acids for X-ray crystal-structure and function studies. Chem Biodiversity, 2010, 7(4): 753–785
Salon J, Sheng J, Jiang J, Chen G, Caton-Williams J, Huang Z. Oxygen replacement with selenium at the thymidine 4-position for the Se base pairing and crystal structure studies. J Am Chem Soc, 2007, 129(16): 4862–4863
Salon J, Chen G, Portilla Y, Germann MW, Huang Z. Synthesis of a 2′-Se-uridine phosphoramidite and its incorporation into oligonucleotides for structural study. Org Lett, 2005, 7(25): 5645–5648
Hassan AE, Sheng J, Zhang W, Huang Z. High fidelity of base pairing by 2-selenothymidine in DNA. J Am Chem Soc, 2010, 132(7): 2120–2121
Hassan AE, Sheng J, Jiang J, Zhang W, Huang Z. Synthesis and crystallographic analysis of 5-Se-thymidine DNAs. Org Lett, 2009, 11(12): 2503–2506
Sheng J, Jiang J, Salon J, Huang Z. Synthesis of a 2′-Se-thymidine phosphoramidite and its incorporation into oligonucleotides for crystal structure study. Org Lett, 2007, 9(5): 749–752
Zhang W, Hassan EA, Huang Z. Synthesis of novel di-Se-containing thymidine and Se-DNAs for structure and function studies. Sci China Chem, 2013, 56(3): 273–278
Sun H, Jiang S, Caton-Williams J, Liu H, Huang Z. 2-Selenouridine triphosphate synthesis and Se-RNA transcription. RNA, 2013, 19 1309–1314
Lin L, Sheng J, Momin RK, Liu H, Huang Z. Facile synthesis and anti-tumor cell activity of Se-containing nucleosides. Nucleosides, Nucleotides Nucleic Acids, 2009, 28(1): 56–66
Brandt G, Carrasco N, Huang Z. Efficient substrate cleavage catalyzed by hammerhead ribozymes derivatized with selenium for X-ray crystallography. Biochemistry, 2006, 45(29): 8972–8977
Carrasco N, Ginsburg D, Du Q, Huang Z. Synthesis of selenium-derivatized nucleosides and oligonucleotides for X-ray crystallography. Nucleosides, Nucleotides Nucleic Acids, 2001, 20(9): 1723–1734
Longworth J, Rahn R, Shulman R. Luminescence of pyrimidines, purines, nucleosides, and nucleotides at 77 K. The effect of ionization and tautomerization. J Chem Phys, 1966, 45: 2930
Walaas E. Fluorescence of adenine and inosine nucleotides. Acta Chem Scand, 1963, 17: 461–463
Börresen H. On the luminescence properties of some purines and pyrimidines. Acta Chem Scand, 1963, 17(4): 921–929
Cohen BJ, Goodman L. Luminescence of purines 1. J Am Chem Soc, 1965, 87(23): 5487–5490
Korshun VA, Manasova EV, Balakin KV, Malakhov AD, Perepelov AV, Sokolova TA, Berlin Yu A. New fluorescent nucleoside derivatives-5-alkynylated 2-deoxyuridines. Nucleosides Nucleotides, 1998, 17(9-11): 1809–1812
Kovaliov M, Segal M, Fischer B. Fluorescent p-substituted-phenyl-imidazolo-cytidine analogues. Tetrahedron, 2013, 69(18): 3698–3705
Bag SS, Saito Y, Hanawa K, Kodate S, Suzuka I, Saito I. Intelligent fluorescent nucleoside in sensing cytosine base: Importance of hydrophobic nature of perylene fluorophore. Bioorg Med Chem Lett, 2006, 16(24): 6338–6341
Ben Gaied N, Glasser N, Ramalanjaona N, Beltz H, Wolff P, Marquet R, Burger A, Mély Y. 8-Vinyl-deoxyadenosine, an alternative fluorescent nucleoside analog to 2′-deoxyribosyl-2-aminopurine with improved properties. Nucleic Acids Res, 2005, 33(3): 1031–1039
Wilson JN, Gao J, Kool ET. Fluorescent nucleoside analogs: Synthesis, properties and applications. Tetrahedron, 2007, 63(17): 3415
Xie Y, Maxson T, Tor Y. Fluorescent nucleoside analogue displays enhanced emission upon pairing with guanine. Org Biomol Chem, 2010, 8(22): 5053–5055
Sinkeldam RW, Greco NJ, Tor Y. Fluorescent analogs of biomolecular building blocks: Design, properties and applications. Chem Rev, 2010, 110(5): 2579–2619
Dodd D, Hudson R. Intrinsically fluorescent base-discriminating nucleoside analogs. Mini-Rev Org Chem, 2009, 6(4): 378–391
Akimitsu O, Yoshio S, Isao S. Design of base-discriminating fluorescent nucleosides. J Photochem Photobiol, C, 2005, 6, 108–122
Salon J, Jiang J, Sheng J, Gerlits OO, Huang Z. Derivatization of DNAs with selenium at 6-position of guanine for function and crystal structure studies. Nucleic Acids Res, 2008, 36(22): 7009–7018
Milne GH, Townsend LB. Synthesis and antitumor activity of alpha- and beta-2′-deoxy-6-selenoguanosine and certain related derivatives. J Med Chem, 1974, 17(3): 263–268
Chu S-H, Davidson DD. Potential antitumor agents. 2. Alpha- and Beta-2′-deoxy-6-selenoguanosine and related compounds. J Med Chem, 1972, 15(10): 1088–1089
Chu SH. Potential antitumor agents. Selenoguanosine and related compounds. J Med Chem, 1971, 14(3): 254–255
Beltagy Y, Waugh W, Repta A. Antioxidants in purification, stabilization, and formulation of the antineoplastic agent 6-selenoguanosine. J Pharm Sci, 1980, 69(10): 1168–1170
Harris NJ, Booth PJ. Folding and stability of membrane transport proteins in vitro. Biochim Biophys Acta, Biomembr, 2012, 1818(4): 1055–1066
Charalambous K, Booth PJ, Woscholski R, Seddon JM, Templer RH, Law RV, Barter LM, Ces O. Engineering de novo membrane-mediated protein-protein communication networks. J Am Chem Soc, 2012, 134(13): 5746–5749
Werten P, Rémigy HW, de Groot B, Fotiadis D, Philippsen A, Stahlberg H, Grubmüller H, Engel A. Progress in the analysis of membrane protein structure and function. FEBS Lett, 2002, 529(1): 65–72
Li F, Xia Y, Meiler J, Ferguson-Miller S. Characterization and modeling of the oligomeric state and ligand binding behavior of purified translocator protein 18 KDa from rhodobacter sphaeroides. Biochemistry, 2013, 54(34): 5884–5899
Rask-Andersen M, Masuram S, Fredriksson R, Schiöth HB. Solute carriers as drug targets: Current use, clinical trials and prospective. Mol Aspects Med, 2013, 34(2): 702–710
Lau FW, Bowie JU. A method for assessing the stability of a membrane protein. Biochemistry, 1997, 36(19): 5884–5892
Hebling CM, Morgan CR, Stafford DW, Jorgenson JW, Rand KD, Engen JR. Conformational analysis of membrane proteins in phospholipid bilayer nanodiscs by hydrogen exchange mass spectrometry. Anal Chem, 2010, 82(13): 5415–5419
Grewer C, Gameiro A, Mager T, Fendler K. Electrophysiological characterization of membrane transport proteins. Annu rev biophys, 2013, 42: 95–120
Shaikh SA, Li J, Enkavi G, Wen PC, Huang Z, Tajkhorshid E. Visualizing functional motions of membrane transporters with molecular dynamics simulations. Biochemistry, 2013, 52(4): 569–587
Teale F. The ultraviolet fluorescence of proteins in neutral solution. Biochem J, 1960, 76(2): 381–388
Alhambra C, Luque F, Estelrich J, Orozco Modesto. Tautomerism of neutral and protonated 6-thioguanine in the gas phase and in aqueous solution. An ab initio study. J Org Chem, 1995, 60(4): 969–976
Leszczynski J. Tautomers of 6-thioguanine: Structures and properties. J Phys Chem, 1993, 97(14): 3520–3524
Stewart MJ, Leszczynski J, Rubin YV, Blagoi YP. Tautomerism of thioguanine: From gas phase to DNA. J Phys Chem A, 1997, 101(26): 4753–4760
Venkateswarlu D, Leszczynski J. Tautomerism and proton transfer in 6-selenoguanine: A post Hartree-Fock level ab initio SCF-MO investigation. J Phys Chem A, 1998, 102(30): 6161–6166
Leszczyński J. Guanine, 6-thioguanine and 6-selenoguanine: Ab initio HF/DZP and MP2/DZP comparative studies. J Mol Struct, 1994, 311: 37–44
Cho HY, Woo SK, Hwang GT. Synthesis and photophysical study of 2′-deoxyuridines labeled with fluorene derivatives. Molecules, 2012, 17(10): 12061–12071
Yoshio S, Azusa S, Shinya I, Isao S. Synthesis and photophysical properties of novel push-pull-type solvatochromic 7-deaza-2′-deoxypurine nucleosides. Tetrahedron Lett, 2011, 52(37): 4726–4729
Callis PR. Electronic states and luminescence of nucleic acid systems. Annu Rev Phys Chem, 1983, 34(1): 329–357
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kaur, M., Huang, Z. Synthesis and optical behaviors of 6-seleno-deoxyguanosine. Sci. China Chem. 57, 314–321 (2014). https://doi.org/10.1007/s11426-013-5038-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-013-5038-y