Abstract
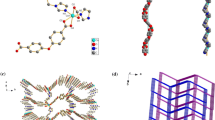
Chiral Schiff-base ligand L was synthesized through six steps in good overall yield from readily available 2-tert-butylphenol and was used to construct one chiral porous metal-metallosalen framework, [Zn5(µ3-OH)2(ZnL)4(H2O)2]·18H2O (1, L = 5′,5″-(1E, 1′E)-(1R, 2R)-cyclohexane-1,2-diylbis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)bis(3′-tert-butyl-4′-hydroxybiphenyl-4-carboxylic acid), under mild reaction conditions. 1 was characterized by IR, TGA, CD, UV, PL, single-crystal and powder X-ray crystallography. The structure of 1 displays a 3-fold interpenetrating 3D framework with 1D channel of 1.14 nm × 0.58 nm and imparts unique Zn(salen) units on the surface of the pore, in which (ZnL)2 dimer acts as multi-functionlized metalloligand. 1 is thermally robust with network decomposition temperature of 400 °C and it also exhibits strong photoluminescence in the visible region.
Similar content being viewed by others
References
Eddaoudi M, David BM, Li HL, Chen BL, Reineke TM, O’Keeffe M, Yaghi OM. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc Chem Res, 2001, 34: 319–330
Qiu SL, Zhu GS. Molecular engineering for synthesizing novel structures of metal-organic frameworks with multifunctional properties. Coord Chem Rev, 2009, 253: 2891–2911
Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O’Keeffe M, Yaghi OM. Hydrogen storage in microporous metal-organic frameworks. Science, 2003, 300: 1127–1129
Lee JY, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Metal-organic framework materials as catalysts. Chem Soc Rev, 2009, 38: 1450–1459
Murray LJ, Dincă M, Long JR. Hydrogen storage in metal-organic frameworks. Chem Soc Rev, 2009, 38: 1294–1314
Chen B, Xiang S, Qian, G. Metal-organic frameworks with functional pores for recognition of small molecules. Acc Chem Res, 2010, 43: 1115–1124
Li JR, Kuppler RJ, Zhou HC. Selective gas adsorption and separation in metal-organic frameworks. Chem Soc Rev, 2009, 38: 1477–1504
Allendorf MD, Bauer CA, Bhakta RK, Houk RJT. Luminescent metal-organic frameworks. Chem Soc Rev, 2009, 38: 1330–1352
Cui Y, Yue Y, Qian G, Chen B. Luminescent functional metal-organic frameworks. Chem Rev, 2011, 112: 1126–1162
Cao AM, Hu JS, Wan LJ. Morphology control and shape evolution in 3D hierarchical superstructures. Sci China Chem, 2012, 55: 2249–2256
Ma L, Abney C, Lin W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem Soc Rev, 2009, 38: 1248–1256
Ma L, Falkowski JM, Abney C, Lin W. A series of isoreticular chiral metal-organic frameworks as a tunable platform for asymmetric catalysis. Nat Chem, 2010, 2: 838–846
Xuan W, Zhu C, Liu Y, Cui Y. Mesoporous metal-organic framework materials. Chem Soc Rev, 2012, 41: 1677–1695
Furukawa H, Ko N, Go YB, Aratani N, Choi SB, Choi E, Yazaydin AÖ, Snurr RQ, O’Keeffe M, Kim J, Yaghi OM. Ultrahigh porosity in metal-organic frameworks. Science, 2010, 329: 424–428
Zhao D, Timmons DJ, Yuan D, Zhou HC. Tuning the topology and functionality of metal-organic frameworks by ligand design. Acc Chem Res, 2010, 44: 123–133
Cho S-H, Ma B, Nguyen ST, Hupp JT. Albrecht-Schmitt TE. A metal-organic framework material that functions as an enantioselective catalyst for olefin epoxidation. Chem Comm, 2006, 24: 2563–2565
Shultz AM, Sarjeant AA, Farha OK, Hupp JT, Nguyen ST. Post-synthesis modification of a metal-organic framework to form metallosalen-containing MOF materials. J Am Chem Soc, 2011, 133: 13252–13255
Song F, Wang C, Falkowski JM, Ma L, Lin W. Isoreticular chiral metal-organic frameworks for asymmetric alkene epoxidation: Tuning catalytic activity by controlling framework catenation and varying open channel sizes. J Am Chem Soc, 2010, 132: 15390–15398
Yuan G, Zhu C, Liu Y, Cui Y. Nano- and microcrystals of a Mn-based metal-oligomer framework showing size-dependent magnetic resonance behaviors. Chem Comm, 2011, 47: 3180–3182
Li G, Yu WB, N J, Liu TF, Liu Y, Sheng EH, Cui Y. Self-assembly of a homochiral nanoscale metallacycle from a metallosalen complex for enantioselective separation. Angew Chem Int Ed, 2008, 47: 1245–1249
Li G, Yu W, Cui Y. A homochiral nanotubular crystalline framework of metallomacrocycles for enantioselective recognition and separation. J Am Chem Soc, 2008, 130: 4582–4583
Zhu C, Yuan G, Chen X, Yang Z, Cui Y. Chiral nanoporous metal-metallosalen frameworks for hydrolytic kinetic resolution of epoxides. J Am Chem Soc, 2012, 134: 8058–8061
Zhu C, Xuan W, Cui Y. Luminescent microporous metal-metallosalen frameworks with the primitive cubic net. Dalton Trans, 2012, 41: 3928–3932
Sheldrick GM. SHELXTL Version 5.1 Software reference manual. Madison, Wisconsin: Bruker AXS, Inc., 1997
Jeon YM, Heo J, Mirkin CA. Acid-functionalized dissymmetric salen ligands and their manganese(III) complexes. Tetrahedron Lett, 2007, 48: 2591–2595
Jeon YM, Heo J, Mirkin CA. Dynamic interconversion of amorphous microparticles and crystalline rods in salen-based homochiral infinite coordination polymers. J Am Chem Soc, 2007, 129: 7480–7481
Heo J, Jeon, YM, Mirkin CA. Reversible interconversion of homochiral triangular macrocycles and helical coordination polymers. J Am Chem Soc, 2007, 129: 7712–7713
Germain ME, Vargo TR, McClure BA, Rack JJ, Van Patten PG, Odoi M, Knapp MJ. Quenching Mechanism of Zn(Salicylaldimine) by Nitroaromatics. Inorg Chem, 2008, 47: 6203–6211
Spek AL. PLATON, Version 1.62. Utrecht, Nederland: University of Utrecht, 1999
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Peng, Y., Zhu, C. & Cui, Y. Synthesis, structure and property of one porous Zn(salen)-based metal-metallosalen framework. Sci. China Chem. 57, 107–113 (2014). https://doi.org/10.1007/s11426-013-5012-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-013-5012-8