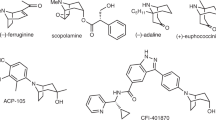
Abstract
A full account of the novel and flexible approach to hydroxylated 8-azabicyclo[3,2,1]octan-3-ones and 9-azabicyclo[3,3,1] nonan-3-ones is presented. Using keto-lactams as the starting materials, this two-step method consists of silyl enol ether formation with TBDMSOTf, lactam activation with Tf2O/DTBMP, and halide-promoted cyclization. Radical dechlorination of the resulting 1-halotropan-3-ones led to the corresponding hydroxylated tropan-3-ones, which can be hydrogenated to yield 3α,6β-dihydroxytropanes. Starting from optically active keto-lactams, the method has been applied to the enantioselective syntheses of (+)-(1S,3S,5R,6S)-pervilleine C (6), (+)-(1S,3R,5S,6R)-valeroidine (3), (+)-(1S,3S,5R,6S)-dibenzoyloxytropane (8), and (+)-(1S,3S,5R,6S)-merredissine (9).
Similar content being viewed by others
References
For selected reviews, see: (a) Christen P. Tropane alkaloids: Old drugs used in modern medicine. In: Attaur-Rahman, Ed. Studies in Natural Products Chemistry. Amsterdam: Elsevier Science, 2000, 22: 717–749
Oliveira SL, da Silva MS, Tavares JF, Sena-Filho JG, Lucena HFS, Romero MAV, Barbosa-Filho JM. Tropane alkaloids from erythroxylum genus: Distribution and compilation of 13C-NMR spectral data. Chem Biodiv, 2010, 7: 302–326
O’Hagan D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat Prod Rep, 2000, 17: 435–446
Grynkiewicz G, Gadzikowska M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol Rep, 2008, 60: 439–463
Zhao LY, Johnson KM, Zhang M, Flippen-Anderson J, Kozikowski AP. Chemical synthesis and pharmacology of 6- and 7-hydroxylated 2-carbomethoxy-3-(p-tolyl)tropanes: Antagonism of cocaine’s locomotor stimulant effects. J Med Chem, 2000, 43: 3283–3294, and reference cited therein
Pei XF, Gupta TH, Badio B, Padgett WL, Daly JW. 6β-Acetoxynortropane: A potent muscarinic agonist with apparent selectivity toward M2-Receptors. J Med Chem, 1998, 41: 2047–2055
Muñoz MA, Muñoz O, Joseph-Nathan P. Absolute configuration determination and conformational analysis of (−)-(3S,6S)-3α,6β-diacetoxytropane using vibrational circular dichroism and DFT techniques. Chirality, 2010, 22: 234–241
Humam M, Christen P, Muñoz O, Hostettmann K, Jeannerat D. Absolute configuration of tropane alkaloids bearing two α,β-unsaturated ester functions using electronic CD spectroscopy: Application to (R,R)-trans-3-hydroxy-senecioyloxy-6-senecioyloxytropane. Chirality, 2008, 20: 20–25
Muñoz MA, Muñoz O, Joseph-Nathan P. Absolute configuration of natural diastereoisomers of 6β-hydroxyhyoscyamine by vibrational circular dichroism. J Nat Prod, 2006, 69: 1335–1340
Muñoz MA, Martínez M, Joseph-Nathan P. Absolute configuration and stereochemical analysis of 3α,6β-dibenzoyloxytropane Muñoz. Phytochem Lett, 2012, 5: 450–454
For a review on the synthetic approaches to enantiomerically pure 8-azabicyclo[3. 2.1]octane derivatives, see: (a) Pollini GP, Benetti S, Risi CD, Zanirato V. Synthetic Approaches to enantiomerically pure 8-azabicyclo[3.2.1]octane derivatives. Chem Rev, 2006, 106: 2434–2454
For a review on the chemistry, design, and structure-activity relationship of cocaine antagonists, see: (b) Singh S. Chemistry, design, and structure-activity relationship of cocaine antagonists. Chem Rev, 2000, 100: 925–1024
For recent synthetic strategies, see: (a) Ding R, Sun BF, Lin GQ. An efficient total synthesis of (−)-Huperzine A. Org Lett, 2012, 14: 4446–4449
Davis FA, Gaddiraju NV, Theddu N, Hummel JR, Kondaveeti SK, Zdilla MJ. Enantioselective synthesis of cocaine C-1 analogues using sulfinimines (N-sulfinyl imines). J Org Chem, 2012, 77: 2345–2359
Shing TKM, So KH. Facile and enantiospecific syntheses of (6S,7R)-6-chloro-7-benzyloxy-, (7S)-halo-, and (7S)-hydroxycocaine and natural (−)-cocaine from D-(−)-ribose. Org Lett, 2011, 13: 2916–2919
Davis FA, Theddu N, Edupuganti R. Asymmetric total synthesis of (S)-(+)-cocaine and the first synthesis of cocaine C-1 analogs from N-sulfinyl β-amino ester ketals. Org Lett, 2010, 12: 4118–4121
For recent enantioselective synthesis of BGT A: (a) Zhang YQ, Liebeskind LS. Organometallic enantiomeric scaffolding: Organometallic chirons. Total synthesis of (−)-Baogongteng A by a molybdenum-mediated [5+2] cycloaddition. J Am Chem Soc, 2006, 128: 465–472
Lin GJ, Zheng X, Huang PQ. A new method for the construction of the hydroxylated tropane skeleton: Enantioselective synthesis of (−)-Bao Gong Teng A. Chem Commun, 2011, 47: 1545–1547
Erycibelline: (c) Zhang ZL, Nakagaw S, Kato A, Jia YM, Hua XG, Yu CY. A concise stereoselective synthesis of (−)-erycibelline. Org Biomol Chem, 2011, 9: 7713–7719
Pervilleine C: (d) Kulkarni K, Zhao AY, Purcell AW, Perlmutter P. The enantioselective total synthesis and unambiguous proof of the absolute stereochemistry of Pervilleine C. Synlett, 2008, 2209–2212
Physoperuvine: (e) Sabine L, Angelika B, Wolfgang F. The CeCl3·nH2O/NaI system in organic synthesis: An efficient water tolerant Lewis acid promoter. Synlett, 2003, 2175–2177
see also: (f) Marek M, Ryszard L. Stereoselective synthesis of tropane alkaloids: Physoperuvine and dihydroxytropanes. Synlett, 1996, 785–786
Yao TR, Chen ZN. Chemical investigation of Chinese medicinal herb, Baogongteng 1. The isolation and preliminary study on a new myotic constituent, Baogongteng A. Acta Pharm Sinica, 1979, 14: 731–735
Rarger G, Martin WF, Mitchell W. The minor alkaloids of Duboisia myoporoides. J Chem Soc, 1937, 1820–1823
Fodor G, Vincze IW, Tóth J. The stereochemistry of the tropane alkaloids. Part XIII. The absolute configuration and a simplified synthesis of valeroidine. J Chem Soc, 1961, 3219–3221
Fodor G, Sóti F. Correlation of valeroidine with S-(−)-methoxysuccinic acid and of mono- and ditigloytropane-3.6-diol with its R-(+)-antimer. Tetrahedron Lett, 1964, 5: 1917–1921
Fodor G, Sóti F. The stereochemistry of the tropane alkaloids. Part XVII. Correlation of valeroidine with S-(-)-methoxysuccinic acid and of mono- and di-tigloyltropane-3,6-diol with its R-(+)-antimer. J Chem Soc, 1965, 6830–6833
Lu Y, Yao TR, Chen ZN. Study on the constituents of erycibe elliptllimba. Acta Pharm Sinica, 1986, 21: 829–835
Humam M, Kehrli T, Jeannerat D, Muñoz O, Hostettmann K, Christen P. Schizanthines N, O, and P, tropane alkaloids from the aerial parts of schizanthus tricolor. J Nat Prod, 2011, 74: 50–53
de Oliveira SL, Tavares JF, Branco MVSC, Lucena HFS, Barbosa-Filho JM, Agra MF, do Nascimento SC, Aguiar JS, da Silva TG, de Simone CA, de Araújo-Júnior JX, da Silva MS. Tropane alkaloids from erythroxylum caatingae plowman. Chem Biodivers, 2011, 8: 155–165
El-Imam YMA, Evans WC, Grout RJ. Alkaloids of erythroxylum cuneatum, E. ecarinatum and E. australe. Phytochemistry, 1988, 27: 2181–2184
Cretton S, Glauser G, Humam M, Jeannerat D, Muñoz O, Maes L, Christen P, Hostettmann K. Isomeric tropane alkaloids from the aerial parts of schizanthus tricolor. J Nat Prod, 2010, 73: 844–847
Humam M, Shoul T, Jeannerat D, Muñoz O, Christen P. Chirality and numbering of substituted tropane alkaloids. Molecules, 2011, 16: 7199–7209
Silva GL, Cui B, Chávez D, You M, Chai H, Rasoanaivo P, Lynn SM, O’Neill MJ, Lewis JA, Besterman JM, Monks A, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. Modulation of the multidrug-resistance phenotype by new tropane alkaloid aromatic esters from erythroxylumpervillei. J Nat Prod, 2001, 64: 1514–1520
Chávez D, Cui B, Chai HB, García R, Mejía M, Norman R, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. Reversal of multidrug resistance by tropane alkaloids from the stems of erythroxylumrotundifolium. J Nat Prod, 2002, 65: 606–610
Chin YW, Jones WP, Waybright TJ, McCloud TG, Rasoanaivo P, Cragg GM, Cassady JM, Kinghorn AD. Tropane aromatic ester alkaloids from a large-scale re-collection of erythroxylumpervillei stem bark obtained in madagascar. J Nat Prod, 2006, 69: 414–417
For a review, see: Dräger B. Chemistry and biology of calystegines. Nat Prod Rep, 2004, 21: 211–223
Mi Q, Cui BL, SilvaDaniel Lantvit GL, Lim E, Chai H, Hollingshead MG, Mayo JG, Kinghorn AD, Pezzuto JM. Pervilleines B and C, new tropane alkaloid aromatic esters that reverse the multidrug-resistance in the hollow fiber assay. Cancer Lett, 2002, 184: 13–20
Jenett-Siems K, Weigl R, Böhm A, Mann P, Tofern-Reblin B, Ott SC, Ghomian A, Kaloga M, Siems K, Witte L, Hilker M, Müller F, Eich E. Chemotaxonomy of the pantropical genus Merremia (Convolvulaceae) based on the distribution of tropane alkaloids. Phytochemistry, 2005, 66: 1448–1464
Niu YY, Zhu L, Cui YY, Liu HZ, Chen HZ, Lu Y. The absolute configuration plays an important role in muscarinic activity of BGT-A and its analogs. Bioorg Med Chem, 2008, 16: 10251–10256
Niu YY, Yang LM, Liu HZ, Cui YY, Zhu L, Feng JM, Yao JH, Chen HZ, Fan BT, Chen ZN, Lu Y. Activity and QSAR study of Baogongteng A and its derivatives as muscarinic agonists. Bioorg Med Chem Lett, 2005, 15: 4814–4818
Yang LM, Wang HN. The preparation and bioactivities of chiral analogs of Baogongteng A. Acta Pharmaceutica Sinica, 1998, 33: 832–835
For preparation of 6′-hydroxytropan-3-one by chemical or enzymatic resolution, see: (a) Yan ZH, Lu Y, Valler A, Liu HZ, Chen NZ. Studies on absolute configuration of analogs of Baogongteng-A. Acta Univ Med Secondae Shanghai, 2001, 21: 199–201
Nicolai C, Sabine L, Angelika B. Enzymatic resolution of tropinone derivatives. Synlett, 2003, 2178–2181
Dai XJ, Huang PQ. A short and flexible synthetic approach to the naturally occurring racemic neoclausenamide and its analogs. Chinese J Chem, 2012, 30: 1953–1956
Wang YH, Ou W, Xie LF, Ye JL, Huang PQ. Towards reaction control: Cis-diastereoselective reductive dehydroxylation of 5-alkyl-4-benzyloxy-5-hydroxy-2-pyrrolidinones. Asian J Org Chem, 2012, 1: 359–365
Chen J, Wang AE, Huo HH, Huang PQ. Recent progress on the total synthesis of natural products in China. Sci China Chem, 2012, 55: 1175–1212
Chen GY, Ye JL, Huang HY, Ruan YP, Wang AE, Huang PQ. Divergent enantioselective synthesis of Rigidiusculamide B and the proposed structure of Rigidiusculamide A: Revision of the relative stereochemistry of Rigidiusculamide A. Chem Asian J, 2012, 7: 504–518
Zhang HK, Li X, Huang H, Huang PQ. Asymmetric syntheses of (8R,8aS)- and (8R,8aR)-8-hydroxy-5-indolizidinones: Two promising oxygenated indolizidine building blocks. Scientia Sinica Chimica, 2011, 41: 732–740 (in Chinese)
Teng B, Zheng JF, Huang HY, Huang PQ. Enantioselective synthesis of Glutarimide alkaloids Cordiarimides A, B, Crotonimides A, B, and Julocrotine. Chinese J Chem, 2011, 29: 1312–1318
Huang SY, Chang Z, Tuo SC, Gao LH, Wang AE, Huang PQ. Versatile construction of functionalized tropane ring systems based on lactam activation: Enantioselective synthesis of (+)-pervilleine B. Chem Commun, 2013, 49: 7088–7090
Xiao KJ, Luo JM, Ye KY, Wang Y, Huang PQ. Direct, One-pot sequential reductive alkylation of Lactams/Amides with Grignard and organolithium reagents through Lactam/Amide activation. Angew Chem Int Ed, 2010, 49: 3037–3040
Liao JC, Xiao KJ, Zheng X, Huang PQ. A concise and divergent approach to Radicamine B and Hyacinthacine A3 based on a step-economic transformation. Tetrahedron, 2012, 68: 5297–5302
Xiao KJ, Wang Y, Ye KY, Huang PQ. Versatile one-pot reductive alkylation of Lactams/Amides via amide activation: Application to the concise syntheses of bioactive alkaloids (±)-Bgugaine, (±)-Coniine, (+)-Preussin, and (−)-Cassine. Chem Eur J, 2010, 16: 12792–12796
Xiao KJ, Wang AE, Huang PQ. Direct Transformation of secondary amides into secondary amines: Triflic anhydride activated reductive alkylation. Angew Chem Int Ed, 2012, 51: 8314–8317
Xiao KJ, Wang AE, Huang YH, Huang PQ. Versatile and direct transformation of secondary amides into ketones via organocerium reagents-based deaminative alkylation. Asian J Org Chem. 2012, 1: 130–132
Xiao KJ, Wang AE, Huang YH, Huang PQ. General direct transformation of secondary amides to ketones via amide activation. Acta Chim Sinica, 2012, 70: 1917–1922
Bélanger G, Dupuis M, Larouche-Gauthier R. Asymmetric total synthesis of (+)-Virosine A via sequential nucleophilic cyclizations onto an activated formamide. J Org Chem, 2012, 77: 3215–3221, and references cited therein.
Gawronski J, Gawronska K. Tartaric and malic acids in synthesis, John Wiley & Sons, Inc.: New York, 1999
Ghosh AK, Koltum ES, Blicer G. Tartaric acid and tartrates in the synthesis of bioactive molecules. Synthesis, 2001, 1281–1301
Huang PQ. Asymmetric synthesis of hydroxylated pyrrolidines, piperidines and related bioactive compounds: from N-acyliminium chemistry to N-α-carbanion chemistry. Synlett, 2006, 1133–1149
Stang PJ, Warren T. Single-Step improved synthesis of primary and other vinyl trifluoromethanesulfonates. Synthesis, 1980, 283–284
For a review on the chemistry of Tf2O, see: (a) Baraznenok IL, Nenajdenko VG, Balenkova ES. Chemical transformations induced by triflic anhydride. Tetrahedron, 2000, 56: 3077–3119
For a paper highlighting recent advancements on the amide activation-based nucleophilic additions, see: (b) Pace V, Holzer W. Chemoselective activation strategies of amidic carbonyls towards nucleophilic reagents. Aust J Chem, 2013, 66: 507–510
Baldwin JE. Rules for ring closure. J Chem Soc Chem Commun, 1976, 734–746
Baldwin JE, Lusch MJ. Rules for ring closure: Application to intramolecular aldol condensations in polyketonic substrates. Tetrahedron, 1982, 38: 2939–2947
For a review on cyclization involving iminium ion intermediates, see: Royer J, Bonin M, Micouin L. Chiral heterocycles by iminium ion cyclization. Chem Rev, 2004, 104: 2311–2352
For related reviews, see: (a) Hall HK Jr, El-Shekeil A. Anti-Bredt bridgehead nitrogen compounds in ring-opening polymerization. Chem Rev, 1983, 83: 549–555
Wamer PM. Strained bridgehead double bonds. Chem Rev, 1989, 89: 1067–1093
Eguchi S, Okano T, Takeuchi H. Synthesis of azamodified adamantane derivatives via bridgehead- and bridge imines. A new aspect in the chemistry of heteroadamantane derivatives. Heterocycles, 1987, 26: 3265–3284
For selected examples, see: (d) Yamazaki N, Suzuki H, Kibayashi C. Nucleophilic alkylation on Anti-Bredt iminium ions. Facile entry to the synthesis of 1-alkylated 2-azabicyclo[3.3.1]nonanes (Morphans) and 5-azatricyclo[6.3.1.01,5]dodecane. J Org Chem, 1997, 62: 8280–8281
Suzuki H, Yamazaki N, Kibayashi C. Synthesis of the azatricyclic core of FR901483 by bridgehead vinylation via an anti-Bredt iminium ion. Tetrahedron Lett, 2001, 42: 3013–3015
Krabbenhoft HO, Wiseman JR, Quinn CB. Bredt’s rule. IX. 9-Methyl-9-azabicyclo[3. 1]non-1-ene. J Am Chem Soc, 1974, 96: 258–259
Wnuk TA, Kovacic P. Participation by adjacent nitrogen during solvolysis of bridgehead halide, 1-chloro-3,3-dimethyl-2-azabicyclo[2.2.2]octane. J Am Chem Soc, 1975, 97: 5807–5810
Majumdar KC, Basu PK, Mukhopadhyay PP. Formation of five- and six-membered heterocyclic rings under radical cyclisation conditions. Tetrahedron, 2004, 60: 6239–6278
Sharp LA, Zard SZ. A short total synthesis of (±)-Aspidospermidine. Org Lett, 2006, 8: 831–834
Ishibashi H, Kato I, Takeda Y, Kogure M, Tamura O. 6-Endo-trig and 5-exo-trig selective aryl radical cyclisations of N-(O-bromobenzyl) enamides. Chem Commun, 2000, 1527
Taniguchi T, Ishita A, Uchiyama M, Tamura O, Muraoka O, Tanabe G, Ishibasi H. 7-Endo selective aryl radical cyclization onto enamides leading to 3-benzazepines: Concise construction of a cephalotaxine skeleton. J Org Chem, 2005, 70: 1922
Taniguchi T, Tanabe G, Muraoka O, Ishibashi H. Total synthesis of (±)-stemonamide and (±)-isostemonamide using a radical cascade. Org Lett, 2008, 10: 197–199
Zaimoku H, Taniguchi T, Ishibashi H. Synthesis of the core of actinophyllic acid using a transannular acyl radical cyclization. Org Lett, 2012, 14: 1656–1658
Poll T, Hady AFA, Karge R, Linz G, Weetman J, Helmchen G. N-substituted hydroxysuccinimides from (S)-malic acid as new reagents for asymmetric Diels-Alder additions to enoates. Tetrahedron Lett, 1989, 30: 5595–5598
For confirmation of racemization free property of this method, see: (b) Zheng JL, Liu H, Zhang YF, Zhao W, Tong JS, Ruan YP, Huang PQ. A study on the racemization step in the synthesis of pyrrolidinols via cyclic α-hydroxyimides. Tetrahedron: Asymmetry, 2011, 22: 257–263
Wijnberg JBPA, Schoemaker HE, Speckamp WN. A regioselective reduction of gem-disubstituted succinimides. Tetrahedron, 1978, 34: 179–187
Bernardi A, Micheli F, Potenza D, Scolastico C, Villa R. Stereoselective synthesis of statin analogues. Tetrahedron Lett, 1990, 31: 4949–4952
Othman RB, Bousquet T, Fousse A, Othman M, Dalla V. Toward improving the chemistry of N-acyliminium ions: nucleophilic substitution reactions of pyrrolidinone derivatives with trialkylsilyl nucleophiles catalyzed by triisopropylsilyltrifluoromethane sulfonate (TIPSOTf). Org Lett, 2005, 7: 2825–2828
For recent reviews on α-amidoalkylation via N-acyliminium ions, see: (b) Yazici A, Pyne SG. Intermolecular addition reactions of N-acyliminium ions (Part I). Synthesis, 2009, 339–368
Yazici A, Pyne SG. Intermolecular addition reactions of N-acyliminium ions (Part II). Synthesis, 2009, 513–541
Renaud P, Seebach D. Enantiomerically pure pyrrolidine derivatives from trans-4-hydroxy-L-proline by electrochemical oxidative decarboxylation and titanium-tetrachloride-mediated reaction with nucleophiles. Helv Chim Acta, 1986, 69: 1704–1710
Sakagami H, Kamikubo T, Ogasawara K. Novel reduction of 3-hydroxypyridine and its use in the enantioselective synthesis of (+)-pseudoconhydrine and (+)-N-methylpseudoconhydrine. J Chem Soc, Chem Commun, 1996, 1433–1434
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, Z., Huang, S., Gao, L. et al. A novel and versatile method for the enantioselective syntheses of tropane alkaloids. Sci. China Chem. 57, 252–264 (2014). https://doi.org/10.1007/s11426-013-4998-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-013-4998-2