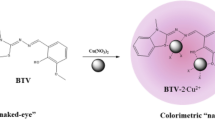
Abstract
By applying an indirect strategy, a new copper (II) complex of a thiosemicarbazone L has been successfully developed as a colorimetric chemosensor for the sensitive detection of mercury (II) ions. In the presence of copper (II) ions, the colorless solution of L became yellow; however, upon the addition of traces of mercury (II) ions, the yellow color faded to colorless immediately. Other ions, including Fe3+, Ag+, Ca2+, Zn2+, Pb2+, Cd2+, Ni2+, Co2+, Cr3+ and Mg2+ had a negligible influence on the probe behavior. The detection limits were 5.0×10−6 M and 3.0×10−7 M of Hg2+ using the visual color changes and UV-vis changes respectively. Test strips based on Cu-L were fabricated, which could act as a convenient and efficient Hg2+ test kits.
Similar content being viewed by others
References
Quang DT, Kim JS. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem Rev, 2010, 110: 6280–6301
Yang YK, Yook KJ, Tae J. A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J Am Chem Soc, 2005, 127: 16760–16761
Song FL, Watanabe SJ, Floreancig PE, Koide K. Oxidation-resistant fluorogenic probe for mercury based on alkyne oxymercuration. J Am Chem Soc, 2008, 130: 16460–16461
Chen C, Wang RY, Guo LQ, Fu NY, Dong HJ, Yuan YF. A squarainebased colorimetric and “turn on” fluorescent sensor for selective detection of Hg2+ in an aqueous medium. Org Lett, 2011, 13: 1162–1165
Yang MH, Thirupathi P, Lee KH. Selective and sensitive ratiometric detection of Hg(II) ions using a simple amino acid based sensor. Org Lett, 2011, 13: 5028–5031
Fang G, Xu MY, Zeng F, Wu SZ. β-cyclodextrin as the vehicle for forming ratiometric mercury ion sensor usable in aqueous media, biological fluids, and live cells. Langmuir, 2010, 26: 17764–17771
Ruan YB, Xie J. Unexpected highly selective fluorescence ‘turn-on’ and ratiometric detection of Hg2+ based on fluorescein platform Tetrahedron, 2011, 67: 8717–8723
Yang Y, Gou XJ, Blecha J, Cao HS. A highly selective pyrene based fluorescent sensor toward Hg2+ detection. Tetrahedron Lett. 2010, 51: 3422–3425
Xua XW, Wang J, Jiao K, Yang XR. Colorimetric detection of mercury ion (Hg2+) based on DNA oligonucleotides and unmodified gold nanoparticles sensing system with a tunable detection range. Biosens Bioelectron, 2009, 24: 3153–3158
Liu YL, Lv X, Zhao Y, Chen ML, Liu J, Wang P, Guo W. A naphthalimiderhodamine ratiometric fluorescent probe for Hg2+ based on fluorescence resonance energy transfer. Dyes Pigments, 2012, 92: 909–915
Wang XY, Su YB, Yang HL, Dong ZP, Ma JT. Highly sensitive fluorescence probe based on chitosan nanoparticle for selective detection of Hg2+ in water. Colloids and Surfaces A: Physicochem Eng Aspects, 2012, 402: 88–93
Zhang YM, Li P, Lin Q, Wei TB, Li JQ. A simple colorimetric sensor with high selectivity for mercury cation in aqueous solution. Phosphorus Sulfur, 2011, 186: 2286–2294
Liu K, Zhou Y, Yao C. A highly sensitive and selective ratiometric and colorimetric sensor for Hg2+ based on a rhodamine nitrobenzoxadiazole conjugate. Inorg Chem Commun, 2011, 14: 1798–1801
Yang XB, Ge JF, Xu QF, Zhu XL, Li NJ, Gu HW, Lu JM. Selective ratiometric detection of Hg2+ in pure water using a phenoxaziniumbased probe. Tetrahedron Lett, 2011, 52: 2492–2495
Zeng DX, Chen Y. A selective, fluorescent probe for Hg2+ detection in aqueous solution. J Photochem Photobiol A, 2007, 186: 121–124
Wang L, Yan JX, Qin W, Liu W, Wang R. A new rhodamine-based single molecule multianalyte (Cu2+, Hg2+) sensor and its application in the biological system. Dyes Pigments, 2012, 92: 1083–1090
Tan H, Zhang Y, Chen Y. Detection of mercury ions (Hg2+) in urine using a terbium chelate fluorescent probe. Sensor Actuat B, 2011, 156: 120–125
Wang HH, Xue L, Yu CL, Qian YY, Jiang H. Rhodamine-based fluorescent sensor for mercury in buffer solution and living cells. Dyes Pigments, 2011, 91: 350–355
Zhang YM, Lin Q, Wei TB, Qin XP, Li Y. A novel smart organogel which could allow a two channel anion response by proton controlled reversible sol-gel transition and color changes. Chem Commun, 2009, 6074–6076
Zhang YM, Lin Q, Wei TB, Wang DD, Yao H, Wang YL. Simple colorimetric sensors with high selectivity for acetate and chloride in aqueous solution. Sens Actuat B, 2009, 137: 447–455
Lin Q, Wei TB, Li Y, Qin XP, Zhang YM. Highly selective colorimetric iodide receptors based on thiosemicarbazides. Sci China Chem, 2009, 39: 357–364
Liu MX, Wei TB, Lin Q, Zhang YM. A novel 5-mercapto triazole Schiff base as a selective chromogenic chemosensor for Cu2+. Spectrochim Acta A, 2011, 79: 1837–1842
Zhang YM, Qin JD, Lin Q, Wei TB. Convenient synthesis and anion recognition properties of N-flurobenzoyl-N′-phenylthioureas in water containing media. J Fluorine Chem, 2006, 127(9): 1222–1227
Li JQ, Wei TB, Lin Q, Li P, Zhang YM. Mercapto thiadiazole based sensor with colorimetric specific selectivity for AcO− in aqueous solution. Spectrochim Acta A, 2011, 83: 187–193
Wei TB, Wang J, Zhang YM. The hydrogen sulfate recognition properties of azo-salicylaldehyde Schiff base receptors. Sci China Chem, 2008, 51: 1051–1056
Li P, Zhang YM, Lin Q, Li JQ, Wei TB. A novel colorimetric HSO4 − sensor in aqueous media. Spectrochim Acta A, 2012, 80: 152–157
Moon SY, Cha NR, Kim YH, Chang SK. New Hg2+ selective chromo- and fluoroionophore based upon 8-hydroxyquinoline. J Org Chem, 2004, 69: 181–183
Song KC, Kim MH, Kim HJ, Chang SK. Hg2+ and Cu2+ selective fluoroionophoric behaviors of a dioxocyclam derivative bearing anthrylacetamide moieties. Tetrahedron Lett, 2007, 48: 7464–7468
Park SM, Kim MH, Choe JI, No KT, Chang SK. Cyclams bearing diametrically disubstituted pyrenes as Cu2+ and Hg2+ selective fluoroionophores. J Org Chem, 2007, 72: 3550–3553
Kim JH, Hwang A-R, Chang SK. Hg2+ selective fluoroionophore of p-tert-butylcalix[4]arene-diaza-crown ether having pyrenylacetamide subunit. Tetrahedron Lett, 2004, 45: 7557–7561
Liu J, Yu M, Wang XC, Zhang Z. A highly selective colorimetric sensor for Hg2+ based on nitrophenyl-aminothiourea. Spectrochim Acta A, 2012, 93: 245–249
Lu M, Ma X, Fan YJ, Fang CJ, Fu XF, Zhao M, Peng SQ, Yan CH. Selective “turn-on” fluorescent chemosensors for Cu2+ based on anthracene. Inorg Chem Commun, 2011, 14: 1864–1867
Wei TB, Li JJ, Lin Q, Yao H, Guo Y, Bai CB, Xie YQ, Zhang YM. Highly selective colorimetric recognition of copper ions based on N-aryl coumarin methyl ketone thiosemicarbazone receptors. Chem J Chinese U, 2012, 33(11): 2452–2456
Hennrich G, Walther W, Resch-Genger U, Sonnenschein H. Cu(II)- and Hg(II)-induced modulation of the fluorescence behavior of a redoxactive sensor molecule. Inorg Chem, 2001, 40: 641–644
Song KC, Kim JS, Park SM, Chung KC, Ahn S, Chang SK. Fluorogenic Hg2+ selective chemodosimeter derived from 8-hydroxyquinoline. Org Lett, 2006, 8(16): 3413–3416
Tsukamoto K, Shinohara Y, Iwasaki S, Maeda H. A coumarin-based fluorescent probe for Hg2+ and Ag+ with an N′-acetylthioureido group as a fluorescence switch. Chem Commun, 2011, 47: 5073–5075
Choi MG, Kim YH, Namgoong JE, Chang SK. Hg2+-selective chromogenic and fluorogenic chemodosimeter based on thiocoumarins. Chem Commun, 2009, 45: 3560–3562
Zhang GX, Zhang DQ, Yin SW, Yang XD, Shuai ZG, Zhu DB. 1, 3-Dithiole-2-thione derivatives featuring an anthracene unit: New selective chemodosimeters for Hg(II) ion. Chem Commun, 2005, 2161–2163
Analytical Methods Committee, Recommendations for the definition, estimation and use of the detection limit. Analyst, 1987, 112: 199–204
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wei, T., Li, J., Bai, C. et al. A highly selective colorimetric sensor for Hg2+ based on a copper (II) complex of thiosemicarbazone in aqueous solutions. Sci. China Chem. 56, 923–927 (2013). https://doi.org/10.1007/s11426-013-4863-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-013-4863-3