Abstract
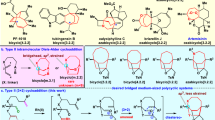
Diversity-oriented synthesis (DOS) has been widely applied in the generation of a large collection of highly functionalized molecules with diverse chemical skeletons. Herein, we report the diversity-oriented synthesis of a series of structurally diverse bicyclic substrates via an efficient tandem conjugate addition/aldol process followed by ring-closing metathesis (RCM). This approach allows us to efficiently prepare a number of structurally complex molecules for the further chemical biology studies.
Similar content being viewed by others
References
For reviews on diversity-oriented synthesis, see: Schreiber SL. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science, 2000, 28(5460): 1964–1969
Burke MD, Schreiber SL. A planning strategy for diversity-oriented synthesis. Angew Chem Int Ed, 2004, 43(1): 46–58
Tan DS. Diversity-oriented synthesis: Exploring the intersections between chemistry and biology. Nat Chem Biol, 2005, 1(2): 74–84
Cordier C, Morton D, Murrison S, Nelson A, O’Leary-Steele C. Natural products as an inspiration in the diversity-oriented synthesis of bioactive compound libraries. Nat Prod Rep, 2008, 25(4): 719–737
O’Connor CJ, Beckmann HSG, Spring DR. Diversity-oriented synthesis: Producing chemical tools for dissecting biology. Chem Soc Rev, 2012, 41(12): 4444–4456
Li H, Wang X, Lei X. Total syntheses of Lycopodium alkaloids (+)-fawcettimine, (+)-fawcettidine, and (−)-8-deoxyserratinine. Angew Chem Int Ed, 2012, 51(2): 491–495
Li C, Tu S, Wen S, Li S, Chang J, Shao F, Lei X. Total synthesis of the G2/M DNA damage checkpoint inhibitor psilostachyin C. J Org Chem, 2011, 76(9): 3566–3570
For recent examples of applying the similar conjugate addition/aldol strategy to total synthesis, see: Arnold LA, Naasz R, Minnaard AJ, Feringa BL. Catalytic enantioselective synthesis of (−)-prostaglandin E1 methyl ester based on a tandem 1,4-addition-aldol reaction. J Org Chem, 2002, 67(21): 7244–7254
Subburaj K, Montgomery J. A new catalytic conjugate addition/aldol strategy that avoids preformed metalated nucleophiles. J Am Chem Soc, 2003, 125(37): 11210–11211
Nicolaou KC, Tang W, Dagneau P, Faraoni R. A catalytic asymmetric three-component 1, 4-addition/aldol reaction: enantioselective synthesis of the spirocyclic system of vannusal A. Angew Chem Int Ed, 2005, 44(25): 3874–3879
Howell GP, Fletcher SP, Geurts K, Horst B, Feringa BL. Catalytic asymmetric synthesis of acyclic arrays by tandem 1,4-addition-aldol reactions. J Am Chem Soc, 2006, 128(46): 14977–14985
Brown MK, Hoveyda AH. Enantioselective total synthesis of clavirolide C. Applications of Cu-catalyzed asymmetric conjugate additions and Ru-catalyzed ring-closing metathesis. J Am Chem Soc, 2008, 130(39): 12904–12906
For a recent example of applying the similar conjugate addition/ aldol/RCM process to total synthesis, see: Dowling MS, Vanderwal CD. Ring-closing metathesis of allylsilanes/electrophilic desilylation to prepare exo-methylidenecycloalkanes. Short syntheses of teucladiol and poitediol. J Am Chem Soc, 2009, 131(42): 15090–15091
For recent reviews of RCM, see: Grubbs RH. Handbook of Metathesis. Weinheim: Wiley, 2003
Lei X, Li H. Selective alkene metathesis in the total synthesis of complex natural product. Top Curr Chem, 2012, 327: 163–196
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, C., Li, X., Wang, X. et al. Diversity-oriented synthesis of bicyclic ring systems via a conjugate addition/aldol/RCM process. Sci. China Chem. 56, 337–341 (2013). https://doi.org/10.1007/s11426-012-4805-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-012-4805-5