Abstract
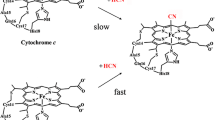
The rebinding kinetics of an amino acid ligand to ferrous microperoxidase-11 (MP11) after photolysis of aggregated ferrous MP11 was measured in aqueous solution with femtosecond transient visible absorption spectroscopy. The kinetics of CO rebinding to ferrous MP11 after photolysis of MP11CO was also measured in aqueous solution with femtosecond transient visible absorption spectroscopy. From these measurements, we found that either Val-11 or Lys-13 rebinds to ferrous MP11 exponentially with an 8 picosecond time constant in aggregated ferrous MP11 solution and that CO rebinds to ferrous MP11 nonexponentially with subnanosecond time scale in MP11CO solution. The kinetics of both the amino acid and CO rebinding to ferrous MP11 in MP11 system mimics that in carbon monoxide oxidation activator protein (CooA) or carboxymethyl cytochrome c (CmCytC) system. We also measured the kinetics of CO rebinding to ferrous MP11 in aqueous solution at different MP11CO concentrations and found that MP11CO concentration has an obvious effect on the kinetics of CO rebinding to ferrous MP11, where both the germinate yield and rate of CO rebinding to ferrous MP11 increase with the increase of MP11CO concentration. These findings suggested that the picosecond amino acid ligand rebinding process could disturb the proximal heme-ligand structure that possibly leads to the subnanosecond CO rebinding kinetics in MP11CO, CooACO and CmCytCCO systems.
Similar content being viewed by others
References
Austin RH, Beeson KW, Eisenstein L, Frauenfelder H, Gunsalus IC. Dynamics of ligand binding to myoglobin. Biochemistry, 1975, 14: 5355–5373
Henry ER, Sommer JH, Hofrichter J, Eaton WA. Geminate recombination of carbon monoxide to myoglobin. J Mol Biol, 1983, 166: 443–451
Ansari A, Jones CM, Henry ER, Hofrichter J, Eaton WA. The role of solvent viscosity in the dynamics of protein conformational changes. Science, 1992, 256: 1796–1798
Balasubramanian S, Lambright DG, Boxer SG. Perturbations of the distal heme pocket in human myoglobin mutants probed by Infrared-spectroscopy of bound CO — Correlation with ligand-binding kinetics. Proc Natl Acad Sci USA, 1993, 90: 4718–4722
Balasubramanian S, Lambright DG, Marden MC, Boxer SG. Co recombination to human myoglobin mutants in glycerol water solutions. Biochemistry, 1993, 32: 2202–2212
Bourgeois D, Vallone B, Schotte F, Arcovito A, Miele AE, Sciara G, Wulff M, Anfinrud P, Brunori M. Complex landscape of protein structural dynamics unveiled by nanosecond Laue crystallography. Proc Natl Acad Sci USA, 2003, 100: 8704–8709
Brunori M, Vallone B, Cutruzzola F, Travaglini-Allocatelli C, Berendzen J, Chu K, Sweet RM, Schlichting I. The role of cavities in protein dynamics: Crystal structure of a photolytic intermediate of a mutant myoglobin. Proc Natl Acad Sci USA, 2000, 97: 2058–2063
Cao WX, Ye X, Georgiev GY, Berezhna S, Sjodin T, Demidov AA, Wang W, Sage JT, Champion PM. Proximal and distal influences on ligand binding kinetics in microperoxidase and heme model compounds. Biochemistry, 2004, 43: 7017–7027
Cao WX, Ye X, Sjodin T, Christian JF, Demidov AA, Berezhna S, Wang W, Barrick D, Sage JT, Champion PM. Investigations of photolysis and rebinding kinetics in myoglobin using proximal ligand replacements. Biochemistry, 2004, 43: 11109–11117
Carver TE, Rohlfs RJ, Olson JS, Gibson QH, Blackmore RS, Springer BA, Sligar SG. Analysis of the kinetic barriers for ligand binding to sperm whale myoglobin using site-directed mutagenesis and laser photolysis techniques. J Biol Chem, 1990, 265: 20007–20020
Franzen S. Spin-dependent mechanism for diatomic ligand binding to heme. Proc Natl Acad Sci USA, 2002, 99: 16754–16759
Franzen S. Carbonmonoxy rebinding kinetics in H93G myoglobin: separation of proximal and distal side effects. J Phys Chem B, 2002, 106: 4533–4542
Franzen S, Fritsch K, Brewer SH. Experimental observation of anharmonic coupling of the heme-doming and iron-ligand out-of-plane vibrational modes confirmed by density functional theory. J Phys Chem B, 2002, 106: 11641–11646
Frauenfelder H, Sligar SG, Wolynes PG. The energy landscapes and motions of proteins. Science, 1991, 254: 1598–1603
Huang Y, Marden MC, Lambry JC, Fontaineaupart MP, Pansu R, Martin JL, Poyart C. Photolysis of the histidine-heme-carbon monoxide complex. J Am Chem Soc, 1991, 113: 9141–9144
Ionascu D, Gruia F, Ye X, Yu AC, Rosca F, Beck C, Demidov AA, Olson JS, Champion PM. Temperature-dependent studies of NO recombination to heme and heme proteins. J Am Chem Soc, 2005, 127: 16921–16934
Kholodenko Y, Gooding EA, Dou Y, Ikeda-Saito M, Hochstrasser RM. Heme protein dynamics revealed by geminate nitric oxide recombination in mutants of iron and cobalt myoglobin. Biochemistry, 1999, 38: 5918–5924
Kriegl JM, Nienhaus K, Deng PC, Fuchs J, Nienhaus GU. Ligand dynamics in a protein internal cavity. Proc Natl Acad Sci USA, 2003, 100: 7069–7074
Lambright DG, Balasubramanian S, Boxer SG. Dynamics of protein relaxation in site-specific mutants of human myoglobin. Biochemistry, 1993, 32: 10116–10124
Lee T, Park J, Kim J, Joo S, Lim M. Dynamics of CO rebinding to protoheme in viscous solutions. Bull Korean Chem Soc, 2009, 30: 177–182
Lim MH, Jackson TA, Anfinrud PA. Modulating carbon monoxide binding affinity and kinetics in myoglobin: The roles of the distal histidine and the heme pocket docking site. J Biol Inorg Chem, 1997, 2: 531–536
Lim MH, Jackson TA, Anfinrud PA. Ultrafast rotation and trapping of carbon monoxide dissociated from myoglobin. Nature Struct Biol, 1997, 4: 209–214
Miers JB, Postlewaite JC, Cowen BR, Roemig GR, Lee IYS, Dlott DD. Preexponential-Limited solid state chemistry: Ultrafast rebinding of a heme-ligand complex in a glass or protein matrix. J Chem Phys, 1991, 94: 1825–1836
Miers JB, Postlewaite JC, Zyung T, Chen S, Roemig GR, Wen X, Dlott DD, Szabo A. Diffusion can explain the nonexponential rebinding of carbon monoxide to protoheme. J Chem Phys, 1990, 93: 8771–8776
Nienhaus K, Deng PC, Kriegl JM, Nienhaus GU. Structural dynamics of myoglobin: Spectroscopic and structural characterization of ligand docking sites in myoglobin mutant L29W. Biochemistry, 2003, 42: 9633–9646
Nienhaus K, Deng PC, Kriegl JM, Nienhaus GU. Structural dynamics of myoglobin: Effect of internal cavities on ligand migration and binding. Biochemistry, 2003, 42: 9647–9658
Nienhaus K, Deng PC, Olson JS, Warren JJ, Nienhaus GU. Structural dynamics of myoglobin-Ligand migration and binding in valine 68 mutants. J Biol Chem, 2003, 278: 42532–42544
Olson JS, Phillips GN. Kinetic pathways and barriers for ligand binding to myoglobin. J Biol Chem, 1996, 271: 17593–17596
Park J, Lee T, Lim M. Viscosity-dependent dynamics of CO rebinding to microperoxidase-8 in glycerol/water solution. J Phys Chem B, 2010, 114: 10897–10904
Schotte F, Lim MH, Jackson TA, Smirnov AV, Soman J, Olson JS, Phillips GN, Wulff M, Anfinrud PA. Watching a protein as it functions with 150-ps time-resolved X-ray crystallography. Science, 2003, 300: 1944–1947
Scott EE, Gibson QH, Olson JS. Mapping the pathways for O2 entry into and exit from myoglobin. J Biol Chem, 2001, 276: 5177–5188
Springer BA, Sligar SG, Olson JS, Phillips GN. Mechanisms of ligand recognition in myoglobin. Chem Rev, 1994, 94: 699–714
Steinbach PJ, Ansari A, Berendzen J, Braunstein D, Chu K, Cowen BR, Ehrenstein D, Frauenfelder H, Johnson JB, Lamb DC. Ligand binding to heme proteins: Connection between dynamics and function. Biochemistry, 1991, 30: 3988–4001
Tian WD, Sage JT, Srajer V, Champion PM. Relaxation dynamics of myoglobin in solution. Phys Rev Lett, 1992, 68: 408–411
Traylor TG, Magde D, Taube DJ, Jongeward KA, Bandyopadhyay D, Luo JK, Walda KN. Geminate recombination of carbon monoxide complexes of hemes and heme proteins. J Am Chem Soc, 1992, 114: 417–429
Walda KN, Liu XY, Sharma VS, Magde D. Geminate recombination of diatomic ligands CO, O2 and NO with Myoglobin. Biochemistry, 1994, 33: 2198–2209
Ye X, Yu AC, Georgiev GY, Gruia F, Ionascu D, Cao WX, Sage JT, Champion PM. CO rebinding to protoheme: Investigations of the proximal and distal contributions to the geminate rebinding barrier. J Am Chem Soc, 2005, 127: 5854–5861
Ye X, Ionascu D, Gruia F, Yu AC, Benabbas A, Champion PM. Temperature-dependent heme kinetics with nonexponential binding and barrier relaxation in the absence of protein conformational substates. Proc Natl Acad Sci USA, 2007, 104: 14682–14687
Srajer V, Reinisch L, Champion PM. Protein fluctuations, distributed coupling, and the binding of ligands to heme proteins. J Am Chem Soc, 1988, 110: 6656–6670
Zhang ZY, Benabbas A, Ye X, Yu AC, Champion PM. Measurements of heme relaxation and ligand recombination in strong magnetic fields. J Phys Chem B, 2009, 113: 10923–10933
Marques HM, Perry CB. Hemepeptide models for hemoproteins: The behavior of N-acetylmicroperoxidase-11 in aqueous solution. J Inorg Biochem, 1999, 75: 281–291
Othman S, Desbois A. Resonance Raman investigation of lysine and N-acetylmethionine complexes of ferric and ferrous microperoxidase — Influences of the axial ligation on the heme c structure. Eur Biophys J, 1998, 28: 12–25
Ricoux R, Lecomte S, Policar C, Boucher JL, Mahy JP. Spectroscopic investigation of isonitrile complexes of ferric and ferrous microperoxidase 8. J Inorg Biochem, 2005, 99: 1165–1173
Vashi PR, Marques HM. The coordination of imidazole and substituted pyridines by the hemeoctapeptide N-acetyl-ferromicroperoxidase-8 (Fe(II)NAcMP8). J Inorg Biochem, 2004, 98: 1471–1482
Munro OQ, Marques HM. Heme-peptide models for hemoproteins. 1. Solution chemistry of N-acetylmicroperoxidase-8. Inorg Chem, 1996, 35: 3752–3767
Karunakaran V, Benabbas A, Youn H, Champion PM. Vibrational coherence spectroscopy of the heme domain in the CO-sensing transcriptional activator CooA. J Am Chem Soc, 2011, 133: 18816–18827
Kumazaki S, Nakajima H, Sakaguchi T, Nakagawa E, Shinohara H, Yoshihara K, Aono S. Dissociation and recombination between ligands and heme in a CO-sensing transcriptional activator CooA — A flash photolysis study. J Biol Chem, 2000, 275: 38378–38383
Rubtsov IV, Zhang TQ, Nakajima H, Aono S, Rubtsov GI, Kumazaki S, Yoshihara K. Conformational dynamics of the transcriptional regulator CooA protein studied by subpicosecond mid-infrared vibrational spectroscopy. J Am Chem Soc, 2001, 123: 10056–10062
Kim J, Park J, Lee T, Lim M. Dynamics of ultrafast rebinding of CO to carboxymethyl cytochrome c. J Phys Chem B, 2009, 113: 260–266
Silkstone G, Jasaitis A, Vos MH, Wilson MT. Geminate carbon monoxide rebinding to a c-type haem. Dalton Transactions, 2005, 3489–3494
Silkstone G, Jasaitis A, Wilson MT, Vos MH. Ligand dynamics in an electron transfer protein-Picosecond geminate recombination of carbon monoxide to heme in mutant forms of cytochrome c. J Biol Chem, 2007, 282: 1638–1649
Zhu RX, Li X, Zhao XS, Yu AC. Photophysical properties of Atto655 dye in the presence of guanosine and tryptophan in aqueous solution. J Phys Chem B, 2011, 115: 5001–5007
Li X, Zhu RX, Yu AC, Zhao XS. Ultrafast photoinduced electron transfer between tetramethylrhodamine and guanosine in aqueous solution. J Phys Chem B, 2011, 115: 6265–6271
Sun QF, Lu R, Yu AC. Structural heterogeneity in the collision complex between organic dyes and tryptophan in aqueous solution. J Phys Chem B, 2012, 116: 660–666
Youn H, Conrad M, Chung SY, Roberts GP. Roles of the heme and heme ligands in the activation of CooA, the CO-sensing transcriptional activator. Biochem Biophys Res Commun, 2006, 348: 345–350
Wang W, Ye X, Demidov AA, Rosca F, Sjodin T, Cao WX, Sheeran M, Champion PM. Femtosecond multicolor pump-probe spectroscopy of ferrous cytochrome c. J Phys Chem B, 2000, 104: 10789–10801
Negrerie M, Cianetti S, Vos MH, Martin JL, Kruglik SG. Ultrafast heme dynamics in ferrous versus ferric cytochrome c studied by time-resolved resonance Raman and transient absorption spectroscopy. J Phys Chem B, 2006, 110: 12766–12781
Cianetti S, Negrerie M, Vos MH, Martin JL, Kruglik SG. Photodissociation of heme distal methionine in ferrous cytochrome c revealed by subpicosecond time-resolved resonance Raman spectroscopy. J Am Chem Soc, 2004, 126: 13932–13933
Ye X, Demidov AA, Champion PM. Measurements of the photodissociation quantum yields of MbNO and MbO2 and the vibrational relaxation of the six-coordinate heme species. J Am Chem Soc, 2002, 124: 5914–5924
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, R., Lu, R. & Yu, A. Rebinding kinetics of dissociated amino acid ligand and carbon monoxide to ferrous microperoxidase-11 in aqueous solution. Sci. China Chem. 56, 230–237 (2013). https://doi.org/10.1007/s11426-012-4788-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-012-4788-2