Abstract
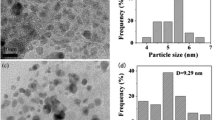
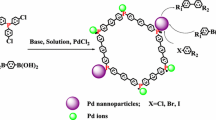
Porous polymer supported palladium catalyst for cross coupling reactions with high activity has been successfully prepared by coordination of Pd2+ species with Schiff bases functionalized porous polymer. The catalyst has been systemically investigated by a series of characterizations such as TEM, N2 adsorption, NMR, IR, XPS, etc. TEM and N2 isotherms show that the sample maintains the nanoporous structure after the modification and coordination. XPS results show that chemical state of palladium species in the catalyst is mainly +2. More importantly, the catalyst shows very high activities and excellent recyclability in a series of coupling reactions including Suzuki, Sonogashira, and Heck reactions. Hot filtration and poison of catalysts experiments have also been performed and the results indicate that soluble active species (mainly Pd(0) species) in-situ generated from the catalyst under the reaction conditions are the active intermediates, which would redeposit to the supporter after the reactions.
Similar content being viewed by others
References
Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev, 1995, 95: 2457–2483
Wu XF, Anbarasan P, Neumann H, Beller M. Palladium-catalyzed carbonylative C-H active of heteroarenes. Angew Chem Int Ed, 2010, 49: 9047–9050
Yin L, Liebscher J. Carbon-carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem Rev, 2007, 107: 133–173 references herein
Schaarachmidt D, Lang H. P,O-ferrocenes in Suzuki-Miyaura C,C couplings. ACS Catal, 2011, 1: 411–416
Lu CH, Chang FC. Polyhedral oligomeric silsesquioxane-encapsulating amorphous palladium nanoclusters as catalysts for Heck reactions. ACS Catal, 2011, 1: 481–488
Fierro-Gonzalez JC, Gates BC. Catalysis by gold dispersed on supports: The importance of cationic gold. Chem Soc Rev, 2008, 37: 2127–2134
Gates BC. Supported metal clusters: Synthesis, structure, and catalysis. Chem Rev, 1995, 95: 511–522
Blaser HU, Indolese A, Schnyder A, Steiner H, Studer M. Supported palladium catalysts for fine chemicals synthesis. J Mol Catal A: Chem, 2001, 173: 3–18
Akiyama R, Kobayashi S. The polymer incarcerated method for the preparation of highly active heterogeneous palladium catalysts. J Am Chem Soc, 2003, 125: 3412–3413
Okamoto K, Akiyama R, Yoshida H, Yoshida T, Kobayashi S. Formation of nanoarchitectures including subnanometer palladium cluster and their use as highly active catalysts. J Am Chem Soc, 2005, 127: 2125–2135
Leadbeater NE, Marco M. Preparation of polymer-supported ligands and metal complexes for use in catalysis. Chem Rev, 2002, 102: 3217–3274
McNamara CA, Dixon MJ, Bradley M. Recoverable catalysts and reagents using recyclable polystyrene-based supports. Chem Rev, 2002, 102: 3275–3300
Ramos ALD, Alves P da S, Aranda DAG, Schmal M. Characterization of carbon supported palladium catalysts: Inference of electronic and particle size effects using rection probes. Appl Catal A, 2004, 277: 71–81
Yu Y, Hu T, Chen X, Xu K, Zhang J, Huang J. Pd nanoparticles on a porous ionic copolymer: A highly active and recyclable for Suzuki-Miyaura reaction under air in water. Chem Commun, 2011, 47: 3592–3594
Liu G, Hou M, Song J, Jiang T, Fan H, Zhang Z, Han B. Immobilization of Pd nanoparticles with functional ionic liquid grafted onto cross-linked polymer for solvebt-free Heck reaction. Green Chem, 2010, 12: 65–69
Djakovitch L, Rollet P. Sonogashira cross-coupling reactions catalysed by copper-free palladium zeolites. Adv Synth Catal, 2004, 346: 1782–1792
Okumura K, Nota K, Yoshida K, Niwa M. Catalytic performance and elution of Pd in the Heck reaction over zeolite-supported Pd cluster catalyst. J Catal, 2005, 231: 245–253
Toebes ML, van Dillen JA, de Jong KP. Synthesis of supported palladium catalysts. J Mol Catal A: Chem, 2001, 173: 75–98
Meanest CP, Weaver DW, Ying JY. Heterogeneous Heck catalysis with palladium-grafted molecular sieves. J Am Chem Soc, 1998, 120: 12289–12296
Djakovitch L, Köhler K. Heck reaction catalyzed by Pd-modified zeolites. J Am Chem Soc, 2001, 123: 5990–5999
Djakovitch L, Köhler K. Heterogeneously catalysed Heck reaction using palladium modified zeolites. J Mol Catal A: Chem, 1999, 142: 275–284
Djakovitch L, Heise H, Köhler K. Heck reactions between aryl halides and olefins catalysed by Pd-complexs entrapped into zeolites NaY. J Organomet Chem, 1999, 584: 16–26
Chang JR, Xu Z, Purnell SK, Gates BC. Synthesis and decarbonylation of platinum carbonyl cluster anions in zeolite NaY made basic by treatment with CsOH. J Mol Catal, 1993, 80: 49–58
Lysén M, Köhler K. Suzuki-miyaura cross-coupling of aryl chlorides in water using ligandless palladium on activated carbon. Synlett, 2005, 11: 1671–1674
Arvela RK, Leadbeater NE. Suzuki coupling of aryl chlorides with phenyboronic acid in water, using microwave heating with simultaneous cooling. Org Lett, 2005, 7: 2101–2104
Zhao F, Bhanage BM, Shirai M, Arai M. Heck reactions of iodobenzene and methy acrylate with conventional supported palladium catalysts in the presence of organic and/or inorganic bases without ligands. Chem Eur J, 2000, 6: 843–848
Hagiwara H, Shimizu Y, Hoshi T, Suzuki T. Ando M. Ohkubo K, Yokoyama C. Heterogeneous Heck reaction catalyzed by Pd/C in ionic liquid. Tetrahedron Lett, 2001, 42: 4349–4351
Zhao F, Shirai M, Arai M. Palladium-catalyzed homogeneous and heterogeneous Heck reactions in NMP and water mixed solvebts using organic, inorganic and mixed bases. J Mol Catal A: Chem, 2000, 154: 39–44
Mori K, Yamaguchi K, Hara T, Mizugaki T, Ebitani K, Kaneda K. Controlled synthesis of hydroxyapatite-supported palladium complexs as highly efficient heterogeneous catalysts. J Am Chem Soc, 2002, 124: 11572–11573
Choudary BM, Madhi S, Chowdari NS, Kantam ML, Sreedhar B. layered double hydroxide supported nanopalladium catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-type coupling reactions of chloroarenes. J Am Chem Soc, 2002, 124: 14127–14136
Parvulescu AN, Hausoul PJC, Bruijnincx PCA, Korhonen ST, Teodorescu C, Klein RJM, Weckhuysen BM. Telomerization of 1,3-butadiene with biomass-derived alcohols over a heterogeneous Pd/TPPTS catalyst based on layered double hydroxides. ACS Catal, 2011, 1: 526–536
Mehnert CP, Weaver DW, Ying JY. Heterogeneous Heck catalysis with palladium-grafted molecular sieves. J Am Chem Soc, 1998, 120: 12289–12296
Crudden CM, Sateesh M, Lewis R. Mercaptopropyl-modified mesoporous silica: A remarkable support for the preparation of a reusable heterogeneous palladium catalyst for coupling reactions. J Am Chem Soc, 2005, 127: 10045–10050
Paetzold E, Oehme G, Fuhrmann H, Richter M, Echelt R, Pohl M-M, Kosslick H. Suzuki reaction over palladium-complex loaded MCM-41 catalysts. Microporous Mesoporous Mater, 2001, 44–45: 537–545
Bedford RB, Singh UG, Walton RI, Williams RT, Davis SA. Nanoparticulate palladium supported by covalently modified silicas: Synthesis, characterization, and application as catalysts for the Suzuki coupling of aryl halides. Chem Mater, 2005, 17: 701–707
Parlett CMA, Bruce DW, Hondow NS, FLee A, Wilson K. Sup port-enhanced selective aerobic alcohol oxidation over Pd/mesopo-rous silicas. ACS Catal, 2011, 1: 636–640
Liu G, Hou M, Wu T, Jiang T, Fan H, Yang G, Han B. Pd(II) immobilized on mesoporous silica by N-heterocyclic carbene ionic liquids and catalysis for hydrogenation. Phys Chem Chem Phys, 2011, 13: 2062–2068
Choi M, Lee DH, Na K, Ryoo R. High catalytic activity of palladium(II)-exchange mesoporous sodalite and NaA zeolite for bulky aryl coupling reactions: Reusability under aerobic conditions. Angew Chem Int Ed, 2009, 48: 3673–3676
Lee DH, Choi M, Yu BW, Yu B-W, Ryoo R. Expand heterogeneous Suzuki-Miyaura coupling reactions of aryl and heteroaryl chlorides under mild conditions. Adv Synth Catal, 2009, 351: 2912–2920
Jin MJ. Tahur A, Kang HJ, Choi M, Ryoo R. Palladium acetate immobilized in a hierarchical MFI zeolite-supported ionic liquid: A highly active and recyclable catalyst for Suzuki reaction in water. Green Chem, 2009, 11: 309–313
Groen JC, Peffer LAA, Moulijin JA, Pérez-Ramírez J. On the introduction of intracrystalline mesoporousity in zeolites upon desilication in alkaline medium. Microporous Mesoporous Mater, 2004, 69: 29–34
Karimi B, Enders D. New N-heterocyclic carbene palladium complex/ionic liquid matrix immobilized on silica: Application as recoverable catalyst for the Heck reaction. Org Lett, 2006, 8: 1237–1240
Zhang Y, Wei S, Liu F, Du Y, Liu S, Ji Y, Yokoi T, Tatsumi T, Xiao F-S. Superhydrophobic nanoporous polymers as efficient adsorbents for organic compounds. Nano Today, 2009, 4: 135–142
Zhang Y, Wei S, Liu F, Nawaz F, Liu S, Zhang H, Xiao F-S. Solvothermal synthesis of carboxyl and amido functionalized mesoporous resins dor water treatmants. J Mater Chem, 2010, 20: 4609–4614
Clark JH, Macquarrie DJ, Mubofu EB. Preparation of a novel silic-supported palladium catalyst and its use in the Heck reaction. Green Chem, 2000, 2: 53–56
Mubofu EB, Clark JH, Macquarrie DJ. A novel Suzuki reaction system based on a supported palladium catalyst. Green Chem, 2001, 3: 23–25
Paul S, Clark JH. A highly active and reusable heterogeneous catalyst for the Suzuki reaction: Synthesis of biaryls and polyaryls. Green Chem, 2003, 5: 635–638
Inbar L, Lapidot A. The structure and biosynthesis of new tetrahydropyrimidine detrivatives in actinomycin D producer Streptomyces parvulus. Use of 13C-and 15N-labeled L-glutamate and 13C and 15N NMR. J Biol Chem, 1988, 263: 16014–16022
Rizeler O, Hennig L, Findeisen M, Welzel P. Search for new moenomycin structure-active relationships synthesis of a trisaccharide precursor of a moenomycin analogue. Tetrahedron Lett, 1997, 53: 1675–1694
Mawhinney DB, Yates Jr JT. FTIR study of the oxidation of amorphous carbon by ozone at 300 K-Direct COOH formation. Carbon, 2001, 39: 1167–1173
Wang L, Meng X, Wang B, Chi W, Xiao F-S. Pyrrolidone-modified SBA-15 supported Au nanoparticles with superior catalytic properties in aerobic oxidation of alcohols. Chem Commum, 2010, 46: 5003–5005
Mubofu EB, Clark JH, Macquarrie DJ. Novel supported heterogeneous palladium catalysts for carbon-carbon forming reactiond. Green Chem, 2000, 3: 23–25
Yang Y, Zhang Y, Hao S, Guan J, Ding D, Shang F, Kan Q. Heterogenization of functionalized Cu(II) and VO(IV) Schiff base complexes by direct immobilization onto amino-modified SBA-15: Styrene oxidation catalysts with H2O2. Appl Catal A: Gen, 2010, 381: 274–218
Mo W, Liu H, Xiong H, Li M, Li G. Preparation of CuCl/1,10-phenanthroline immobilized on polystyrene and catalytic performance in oxidative carbonylation of methanol. Appl Catal A: Gen, 2007, 333: 172–176
Yang H, Han X, Li G, Wang Y. N-Heterocyclic carbene palladium complex supported on ionic liquid-modified SAB-16: an efficient and highly recyclable catalyst for the Suzuki and Heck reactions. Green Chem, 2009, 11: 1184–1193
Adam S, Bauer A, Timpe O, Wild U, Mestl G, Bensch W, Schlögl R. The origin of the positive effect of cadmium acetate on the action of supported palladium catalysts. Chem Eur J, 1998, 4: 1458–1469
Kan C, Huang J, He W, Zhang F. Periodic mesoporous silica-immobilized palladium(II) complex as an effective and reusable catalyst for water-media carbon-carbon coupling reactions. J Organomet Chem, 2010, 695: 120–127
Stevens PD, Fan JD, Gardimalla HMR, Yen M, Gao Y. Superparamagnetic nanoparticle-supported catalysis of Suzuki cross-coupling reactions. Org Lett, 2005, 7: 2085–2088
Sonogashira K, Tohda Y, Hagihara N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett, 1975, 16: 4467–4470
De-la-Rosa MA, Velarde E, Guzmán A. Cross-coupling reactions of monosubstituted acetylenes and aryl halides catalyzed by palladium on charcoal. Synth Commun, 1990, 20: 2059–2064
Li PH, Wang L. An amine-,copper- and phosphine-free sonogashira coupling reaction catalyzed by immobilization of palladium in organic-inorganic hybrid materials. Adv Synth Catal, 2006, 348: 681–685
Fulmer DA, Shearouse WC, Medonza ST, Mack J. Solvent-free Sonogashira coupling reaction via high speed ball milling. Green Chem, 2009, 11: 1821–1825
Karimi B, Akhavan PF. A study on applications of N-substituted main-chain NHC-palladium polymers as recyclable self-supported catalysts for the Suzuki-miyaura coupling of aryl chlorides in water. Inorg Chem, 2011, 50: 6063–6072
Richardson JM, Jones CW. Poly(4-vinylpyridine) and quadrapure TU as selective poisons for soluble catalytic species in palladium-catalyzed coupling reactions-application to leaching from polymer-entrapped palladium. Adv Synth Catal, 2006, 348: 1207–1216
Klingelhofer S, Heitz W, Greiner A. Preparation of palladium colloids in block copolymer micelles and their use for the catalysis of the Heck reaction. J Am Chem Soc, 1997, 119: 10116–10120
Karimi B, Elhamifar D, Clark JH, Hunt AJ. Ordered mesoporous organosilica with ionic-liquid framework: An efficient and reusable support for the palladium-catalyzed Suzuki-Miyaura coupling reaction in water. Chem Eur J, 2010, 16: 8047–8053
Richardson JM, Jones CW. Strong evidence of solution-phase catalysis associated with palladium leaching from immobilized thiols during Heck and Suzuki coupling of aryl iodides, bromides, and chlorides. J Catal, 2007, 251: 80–93
Crudden CM, Sateesh M, Lewis R. Mercaptopropyl-modified mesoporous silica: A remarkable support for the preparation of a reusable, heterogeneous palladium catalyst for coupling reactions. J Am Chem Soc, 2005, 127: 10045–10050
Karimi B, Zareyee D. Design of a highly efficient and water-tolerant sulfonic acid nanoreactor based on tunable ordered porous silica for the von pechmann reaction. Org Lett, 2008, 10: 3989–3992
Hankari SE, Kadib AE, Finiels A, Bouhaouss A, Moreau JJE, Crudden CM, Brunel D, Hesemann P. SBA-15-Type organosilica with 4-mercapto-N,N-bis-(3-Si-propyl) butanamide for palladium scavenging and cross-coupling catalysis. Chem Eur J, 2011, 17: 8984–8994
Yu K, Sommer W, Richardson JM, Weck M, Jones CW. Evidence that SCS Pincer Pd(II) complexes are only precatalysts in Heck catalysis and the implications for catalyst recovery and reuse. Adv Synth Catal, 2005, 347: 161–171
Widegren JA, Finke RG. A review of the problem of distinguishing true homgeneous catalysis from soluble or other metalparticle heterogeneous catalysis under reducing conditions. J Mol Catal A, 2003, 198: 317–341
Consorti CS, Flores FR, Dupont J. Kinetics and mechanistic aspects of the Heck reaction promoted by a CN-palladacycle. J Am Chem Soc, 2005, 127: 12054–12065
Eberhard MR. Insights into the Heck reaction with PCP pincer palla dium(II) complexes. Org Lett, 2004, 6: 2125–2128
Pröckl SS, Kleist W, Gruber MA, Köhler K. In situ generation of highly active dissolved palladium species from sold catalysts-A concept for the activation of aryl chlorides in the Heck reaction. Angew Chem Int Ed, 2004, 43: 1917–1918
Heidenreich RG, Krauter JGE, Pietsch J, Köhler K. Control of Pd leaching in Heck reactions of bromoarenes catalyzed by Pd supported on activated carbon. J Mol Catal A, 2002, 182–183: 499–509
MauQuarrie S, Horton JH, Barnes J, McEkeney K, Loock H-P, Crudden CM. Visual observation of redistribution and dissolution of palladium during the Suzuki-Miyaura reaction. Angew Chem Int Ed, 2008, 47: 3279–3282
Amatore C, Jutand A. Anionic Pd(0) and Pd(II) intermediates in palladium-catalyzed Heck and cross-coupling reactions. Acc Chem Res, 2000, 33: 314–321
Ennis DS, Mcmanus J, Wood-Kaczmar W, Richardson J, Smith GE, Carstais A. Multikilogram-scale synthesis of a biphenyl carboxylic acid derivative using a Pd/C-mediated suzuki coupling approach. Org Process Res Dev, 1999, 3: 248–252
LeBlond CR, Andrews AT, Sun YK, Sowa JR. A ctivation of aryl chlorides for Suzuki cross-coupling by ligandless, heterogeneous palladium. Org Lett, 2001, 3: 1555–1557
Heidenreich RG, Köhler K, Krauter JGE, Pietsch J. Pd/C as a highly active catalyst for Heck, Suzuki and Sonogashira reactions. Syn Lett, 2002, 1118–1201
Conlon DA, Pipik B, Ferdinand S, LeBlond CR, Sowa JR, Izzo B. Suzuki-miyaura cross-coupling with quasi-heterogeneous palladium. Adv Synth Catal, 2003, 345: 931–935
Dyer UC, Shapland PD, Tiffin PD. Preparation of enantiopure 4-arylmandelic acids vis a Pd/C catalysed Suzuki coupling of enantiopure 4-bromomandelic acid. Tetrahedron Lett, 2001, 42: 1765–1767
Albéniz AC, Carrera N. Polymers for green C-C couplings. Eur J Inorg Chem, 2011, 15: 2347–2360
Lamblin M, Nassar-Hardy L, Hierso JC, Fouquet E, Felpin F-X. Recyclable heterogeneous palladium catalysts in pure water: Sustainale developments in Suzuki, Heck, Sonogashira and Tsuji-Trost reactions. Adv Synth Catal, 2010, 352: 33–79
Weck M, Jones CW. Mizoroki-Heck coupling using immobilized molecular precatalysts: Leaching active species from Pd pinceers, entrapped Pd salts, and Pd NHC complexes. Inorg Chem, 2007, 46: 1865–1875
Xi Z, Zhou N, Sun Y, Li K. Reaction-controlled phase-transfer catalysis for propylene epoxidation to propylene oxide. Science, 2001, 292: 1139–1141
Brink GJT, Arends IWCER, Sheldon A. Green, catalytic oxidation of alcohols in water. Science, 2000, 287: 1636–1639
Joo F. Aqueous biphasic hydrogenations. Acc Chem Res, 2002, 35: 738–745
Ooi T, Maruoka K. Recent advances in asymmetric phase-transfer catlysis. Angew Chem Int Ed, 2007, 46: 4222–4266
Leng Y, Wang J, Zhu D. Heteropolyanion-based ionic liquids: Reaction-induced self-separation catalysts for esterification. Angew Chem Int Ed, 2008, 121: 174–177
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sun, Q., Zhu, L., Sun, Z. et al. Porous polymer supported palladium catalyst for cross coupling reactions with high activity and recyclability. Sci. China Chem. 55, 2095–2103 (2012). https://doi.org/10.1007/s11426-011-4491-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-011-4491-8